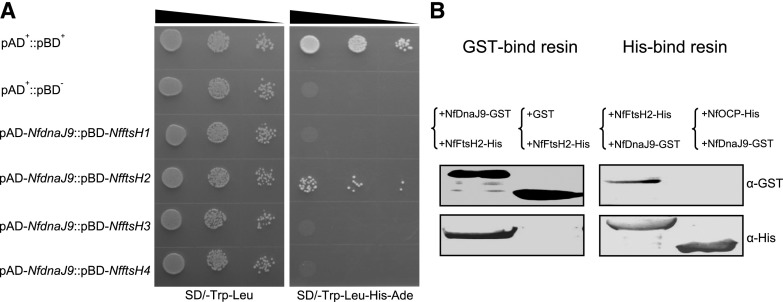
Figure 4.
In vitro interaction between NfDnaJ9 and NfFtsH2. A, Y2H assay of the interaction between NfDnaJ9 and NfFtsH. The yeast transformants (10 μL each of cell suspensions diluted from OD600 0.1 to 0.001, as indicated by the slope of the black triangles from left to right) expressing positive control plasmids (pAD+ and pBD+), negative control plasmids (pAD+ and pBD−) and plasmids for testing protein interaction (pAD-NfdnaJ9 and pBD-NfftsH) were grown on a SD/-Trp-Leu or SD/-Trp-Leu-His-Ade agar plate for 3 d. The yeast strain that grew on the SD/-Trp-Leu plate indicated that two plasmids (pAD and pGB) were cotransformed into S. cerevisiae AH109, and the yeast strain that grew on the SD/-Trp-Leu-His-Ade plate indicated that the two tested proteins interacted in S. cerevisiae AH109. B, Pull-down assay of the interaction between NfDnaJ9 and NfFtsH2. The supernatants of the extracts from GST-tagged NfDnaJ9 or His-tagged NfFtsH2 overexpressed E. coli were mixed at 4°C for overnight, and then were copurified through a GST-binding (left, +NfDnaJ9-GST+NfFtsH2-His) or His-binding resin (right, +NfFtsH2-His+NfDnaJ9-GST). The GST-tag and His-tag signals of copurified proteins were detected with the specific antibodies against His-Tag and GST-Tag, respectively. The samples that GST and His-tagged NfFtsH2 mixture and copurification through a GST-bind resin (left, +GST+NfFtsH2-His), and nonrelative His-tagged NfOCP and GST-tagged NfDnaJ9 mixture and copurification through a His-binding resin (right, +NfOCP-His+NfDnaJ9-GST) were used as negative controls to exclude the nonspecific interactions.