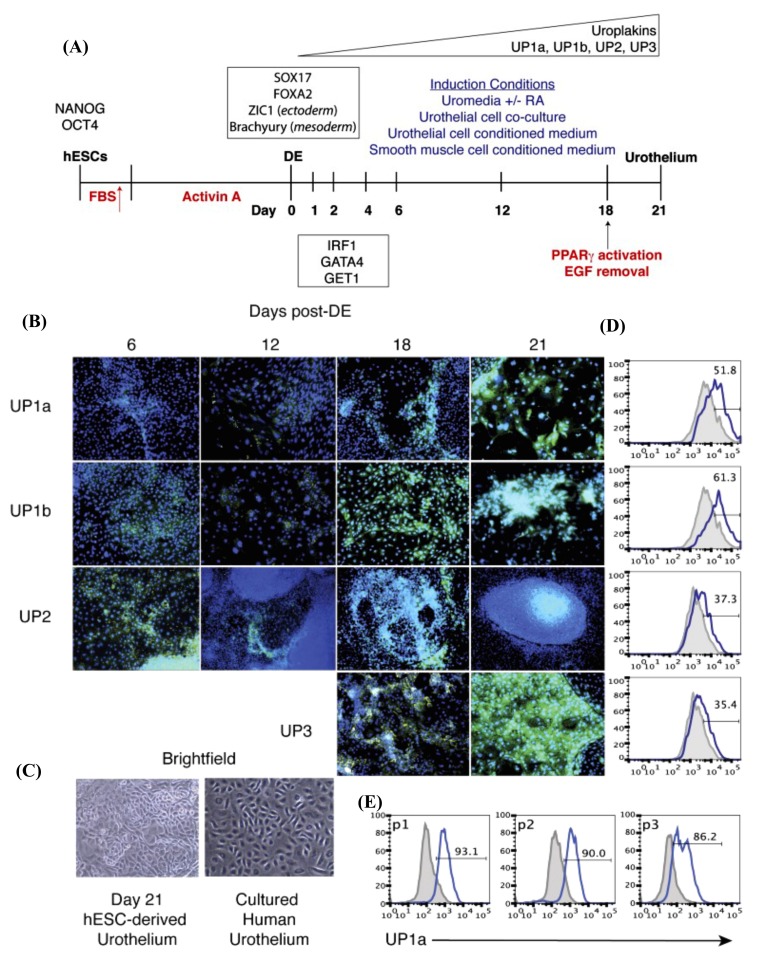
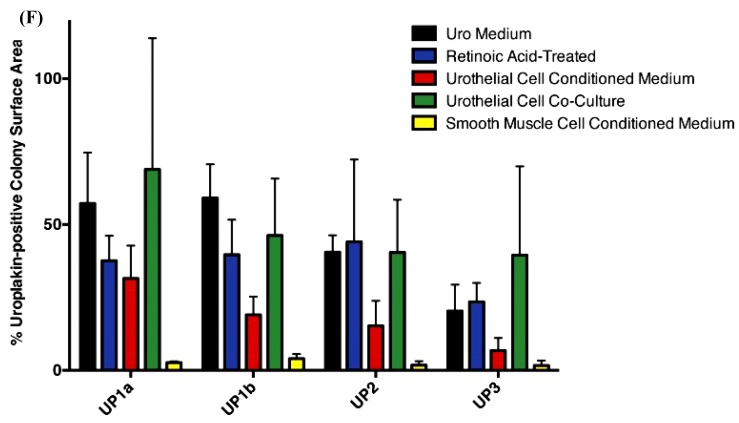
Figure 4.
In vitro urothelium differentiation of hESCs. (A) Schematic diagram illustrating the protocol for urothelium differentiation of hESCs. (B) Intracellular cytokine flow cytometry analysis for the expression of UP subtypes (green color) on days 6, 12, 18, and 21 after DE were analyzed for the expression of all four subtypes of uroplakins (green) by intracellular cytokine flow cytometry. For nuclear counterstaining, DAPI (4′,6-diamidino-2-phenylindole; blue) was used. Magnification, 10×. (C) Images of cultured human urothelium and hESC-derived urothelium using bright-field microscopy on day 21 of the culture. Magnification, 10×. (D) Flow cytometry analysis of UP expression on day 21 of culturing. (E) Flow cytometry analysis for UP1a of H9-derived urothelial cultures at each passage (P 1–3). DE, definitive endoderm. (F) ImageJ-based quantification of the expression of each uroplakin subtype that obtained from the intracellular cytokine flow cytometry data (Figure 4B) from day 21 of culture using the five culture conditions that represented by bars of different colors. The vertical axis of Figure 4D,E represents the relative uroplakin expression amount to the colony surface area that expressed in percentage. hESCs, human embryonic stem cells. DE, definitive endoderm. hESCs, human embryonic stem cells. RA, retinoic acid. EGF, epidermal growth factor. PPARγ, peroxisome proliferator-activated receptor γ. UP, uroplakin. FBS, fetal bovine serum. These figures are reproduced from articles by Osborn et al. 2014 [14] following permission from John Wiley and Sons. In flow cytometry data, the grey histogram denotes the negative control and the solid blue line indicates the positive uroplakin staining. Up arrow (red-colored) represents the treatment of hESCs with a gradual increase in the concentration of FBS for three days that followed by Activin A treatment for nine days. Up arrow (black-colored) indicates the time point (on day 18) of adding PPARγ activator, troglitzone, and removal of EGF for induction of the terminal differentiation.