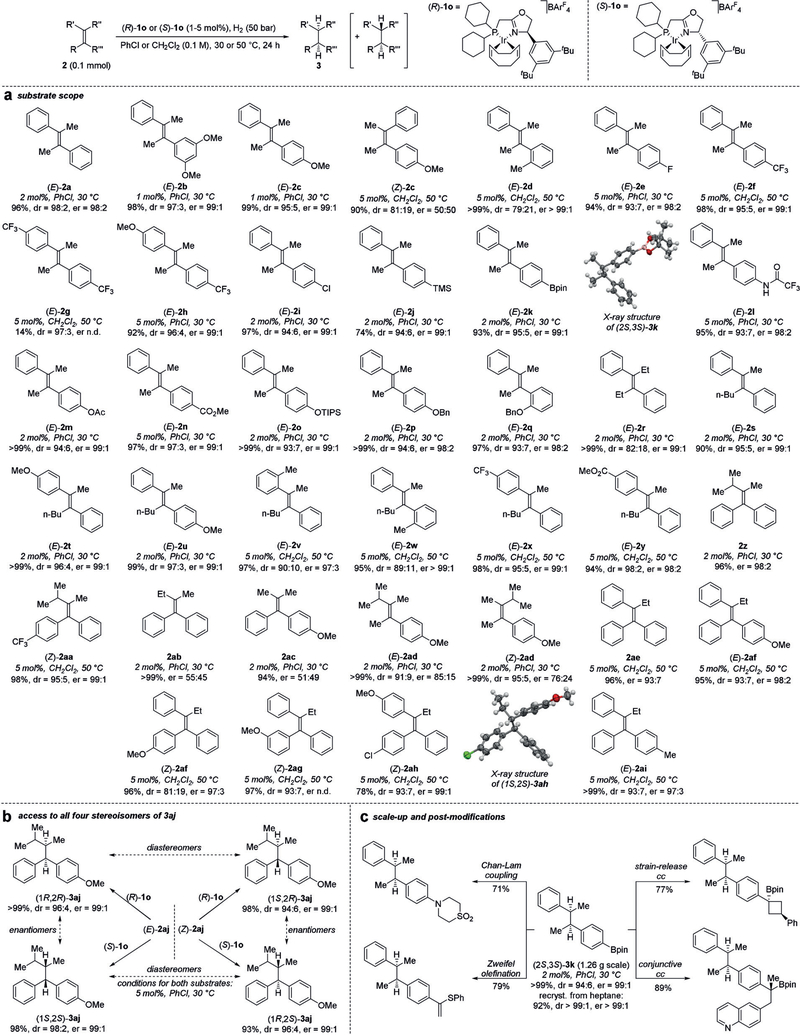
Figure 3.
Ir-catalyzed asymmetric hydrogenation of unfunctionalized tetrasubstituted acyclic olefins. The general reaction scheme is shown at the top. a,b) Yields of isolated products are reported (except for (2S,3S)-3g); dr and er (major diastereomer) are obtained from the crude reaction mixtures after filtration through silica gel. Absolute configurations of products are assigned in analogy to (2S,3S)-3k and (1S,2S)-3ah, for which it was determined by X-ray crystallography.[12] c) Scale-up of the synthesis of (2S,3S)-3k at 1.26 g (3.8 mmol) and post-modifications (see the Supporting Information for details) of (2S,3S)-3k. Yields of isolated post-modification products are shown (dr>20:1 for all isolated products). Ac=acetyl; Bn=benzyl; pin=pinacolato; TIPS=triisopropylsilyl; TMS=trimethylsilyl.