Fig. 1. A group of multipotent sphere-forming stem cells existed in dental papilla.
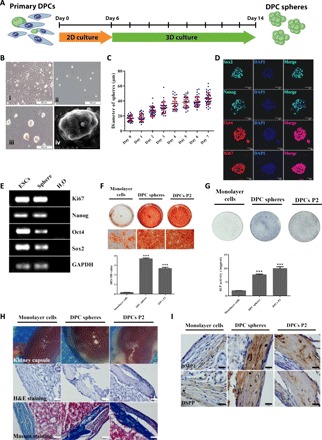
(A) Schematic diagram of the 3D culture assay. DPCs were isolated from tooth germs of neonatal pups and cultured in standard DPC medium for about 1 week before further seeded in MC culture medium to initiate sphere formation. Spheres and control monolayer cells were collected at day 8 for further characterization. (B) Representative images for the typical morphology of primary mouse DPCs P2 (i) and DPC spheres on days 0 (ii) and 7 (iii). Scale bars, 200 μm. Scanning electron microscopy morphology of DPC spheres (iv). Scale bar, 20 μm. (C) 3D culture supported continuous growth of DPC spheres. The growth and diameter of DPC sphere were monitored over time in 3D culture system. (D) DPC spheres were positive for pluripotency and proliferation markers including Oct4, Sox2, Nanog, and Ki67. DPC spheres were collected at day 8 and immunostained with antibodies against Oct4, Sox2, Nanog, and Ki67. DAPI (4′,6-diamidino-2-phenylindole) was used for nuclear staining. Scale bars, 50 μm. (E) Expression of Oct4, Sox2, Nanog, and Ki67 in DPC spheres were also confirmed by RT-PCR. Complementary DNAs from mESCs (mouse embryonic stem cells) were used as the positive control and H2O as the negative control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (F) Alizarin Red S (ARS) staining and quantification indicated DPC sphere–derived cells retained differentiation capabilities compared with 2D monolayer cultures. Monolayer cells, DPC sphere–derived cells, and DPCs at P2 were incubated in osteoinductive medium (OIM) for 10 days, respectively. Sphere-derived cells exhibited enhanced mineralization from monolayer cultures. Error bar represents two independent experiments with triplicates. ***P < 0.001 (Student’s t test). OD, optical density. (G) ALP staining and quantification indicated DPC sphere–derived cells retained differentiation capabilities compared with 2D monolayer cultures. Cells were incubated in OIM for 7 days. Error bar represents two independent experiments with triplicates. ***P < 0.001. (H) Strongly mineralized structures were formed by DPC sphere–derived cells when transplanted in renal capsules. Monolayer cells, DPC sphere–derived cells, and DPCs P2 were implanted into renal capsule of C57BL/6 mice together with Matrigel. Samples were harvested after 4 weeks. The hematoxylin and eosin (H&E) and Masson staining were used to show the mineralized structures. Scale bars, 50 μm. (I) Immunohistochemical analysis of odontogenic markers (DMP1 and DSPP) indicated that DPC sphere–derived cells retained strong potential for odontogenic differentiation. Scale bars, 20 μm.
