Fig. 4. Soft matrix promotes the ability of MSCs to produce and recruit monocytes upon TNFα stimulation.
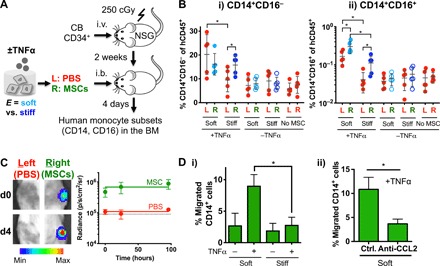
(A) Experimental scheme. Sublethally irradiated (250 cGy) NSG mice were injected intravenously (i.v.) with 20,000 human cord blood HSPCs per 20 g of mouse. After 2 weeks, MSCs that were cultured in soft or stiff gels for 1 day followed by ±TNFα treatment (100 ng/ml) for three additional days were retrieved and delivered to the right (R) tibia by an intrabone (i.b.) route, while the left (L) tibia was delivered with PBS. After 4 days, both tibias were analyzed for human monocyte subsets. (B) Percentage of (i) classical (CD14+CD16−) and (ii) intermediate (CD14+CD16+) human monocytes in tibias. P < 0.05, two-way ANOVA with Sidak’s multiple comparison test, *P < 0.05. n = 5 recipients from two independent experiments. (C) MSCs remain localized in one tibia after intrabone delivery to NSG mice. Firefly luciferase–transduced MSCs were delivered in the right tibia, while PBS was injected in the left tibia, followed by IVIS imaging over 4 days. Left: Representative images at days 0 and 4 after MSC delivery. Scale bar, radiance (p/sec/cm2/sr, min: 1 M, max: 15 M). Right: Quantification of radiance signals over 4 days. The signal from the left tibia remains at the background level (~90,000, dotted line), while that from the right tibia remains ~10-fold higher over the 4 days. (D) TNFα-treated MSCs in soft matrix increase migration of human peripheral blood CD14+ monocytes via secretion of CCL2. MSCs in soft or stiff alginate hydrogel were treated with TNFα (100 ng/ml) for 1 day prior to washout and collection of the media for 1 day for the Transwell migration assay. (i) The extent of CD14+ migration under chemotactic gradient through 3-μm pores for 3 hours. P < 0.05 from one-way ANOVA with Tukey’s post hoc test, *P < 0.05 (n = 3 experiments). (ii) CCL2 is required for increased chemotaxis of CD14+ cells by the media from TNFα-treated MSCs in soft matrix. The media were treated with a neutralizing antibody against CCL2 (anti-CCL2) or isotype control (ctrl) for 3 hours prior to the Transwell assay. *P < 0.05, paired t test (n = 3 experiments). All error bars for both (i) and (ii), ±SEM.
