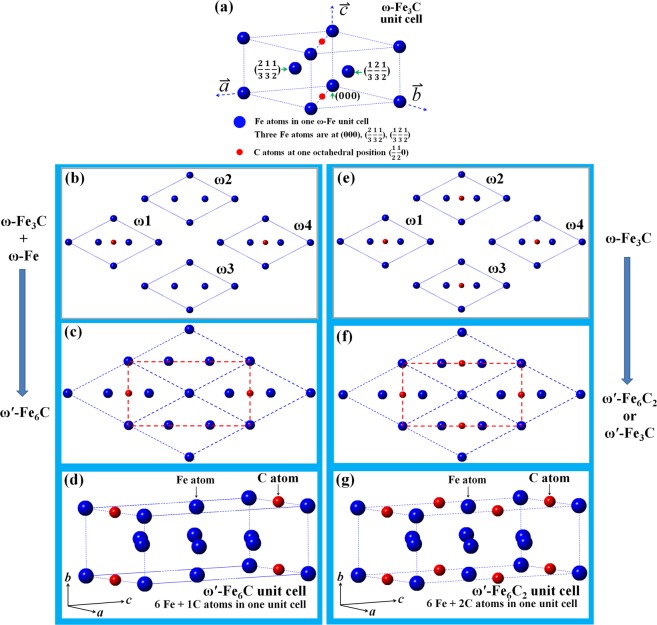
Figure 1.
Atomic structure of various carbides. (a) Unit cell of ω-Fe3C crystal structure. (b) Two ω-Fe3C and two ω-Fe unit cells projected along their c axes. (c) Coarsening of the four unit cells of the ω-Fe3C and ω-Fe in (b) results in the formation of new carbide (ω′-Fe6C) outlined by red dashed lines. (d) The ω′-Fe6C unit cell can have an orthorhombic structure and lattice parameters (aω′ = 4.033 Å, bω′ = 2.47 Å, and cω′ = 6.986 Å for aα-Fe = 2.852 Å), and C atom at (0.5 0 0). (e) Four ω-Fe3C unit cells. (f) Coarsening of the four ω-Fe3C unit cell in (e) results in the formation of a new carbide (ω′-Fe6C2 or ω′-Fe3C) with the same crystal structure and lattice parameters as the ω′-Fe6C. (g) The ω′-Fe6C2 atomic structure in one unit cell.