Figure 12.
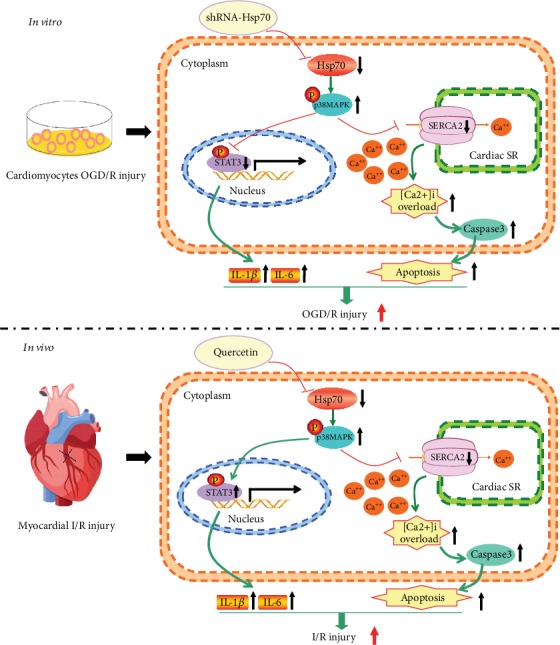
Schematic showing Hsp70 inhibition aggravates cardiac I/R injury through regulating p38 MAPK signaling. In vitro, knockdown of Hsp70 increases p38 MAPK phosphorylation, leading to downregulation of SERCA and phosphorylated STAT3 expression. Decreased SERCA activity causes cytosolic Ca2+ reuptake dysfunction. Increased cytosolic free Ca2+ results in [Ca2+]i overload, triggering caspase-3-dependent cell apoptosis. Besides, decreased STAT3 phosphorylation activates the transcription of IL-1β and IL-6, promoting inflammatory response. In vivo, Hsp70 inhibition enhances the level of p38 MAPK phosphorylation, reduces SERCA activity, and increases STAT3 phosphorylation. Decreased SERCA leads to [Ca2+]i overload and cell apoptosis, and increased STAT3 phosphorylation induces inflammatory response by upregulating IL-1β and IL-6. OGD/R: oxygen-glucose deprivation/reoxygenation; I/R: ischemia/reperfusion; SR: sarcoplasmic reticulum; [Ca2+]i: intracellular calcium.
