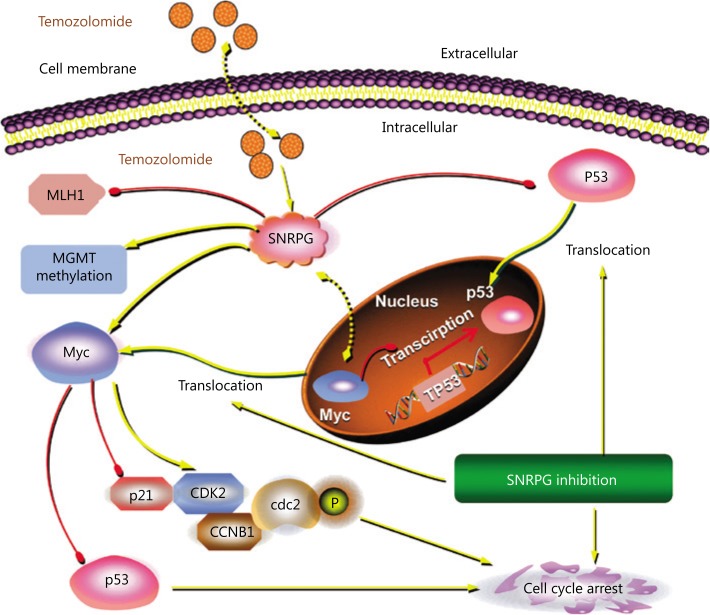
Figure 8.
Schematic diagram of the molecular mechanisms of SNRPG-mediated p53 targeting of TMZ-induced glioblastoma multiforme (GBM) growth inhibition. The SNRPG-mediated inhibitory effect on glioma cells might have been due to the direct suppression of Myc as well as p53 and the ensuing modification of their downstream molecules including p21, CDK2, CCNB1, and cdc2, possibly promoting apoptosis and cell cycle arrest by interacting directly or indirectly with related proteins. Specifically, SNRPG suppression promoted the cytosolic translocalization of Myc, as well as the nuclear translocalization of p53. Furthermore, knockdown of SNRPG in GBM cells promoted the inhibitory effect of TMZ on cell proliferation by targeting Myc through the p53 signaling pathway, suggesting its regulatory effect on TMZ sensitivity. Besides, SNRPG decreased the protein expression of the mismatch repair (MMR) protein MLH1 and increased MGMT methylation, which could all be well-established mechanisms of TMZ resistance. These findings could indicate that SNRPG-driven systemic Myc-regulated p53 activation and sensitization of GBM cells to TMZ are highly promising strategies for treating glioma.