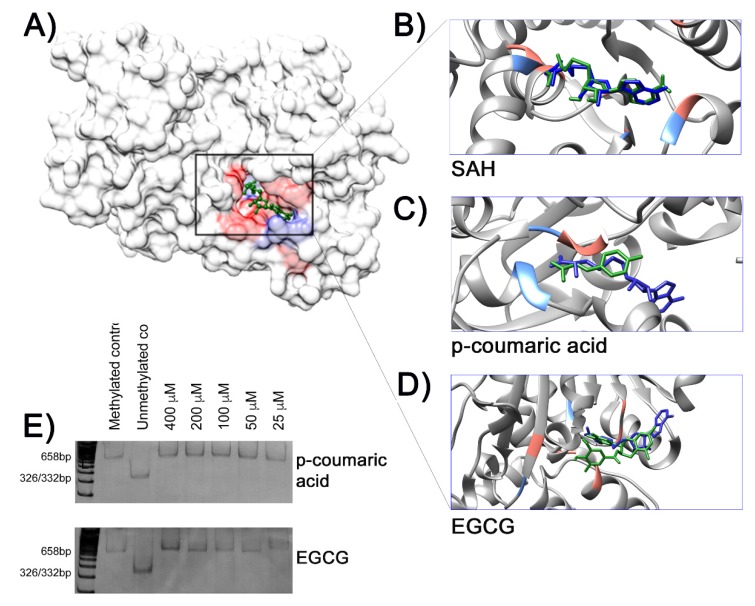
Figure 3.
(A) Crystalografic model of the methyltransferase (MTase) domain of human DNA methyltransferase 1 (DNMT1) (PDBID:4WXX) complexed with S-adenosyl-l-homocisteine (SAH). The highlighted area in the rectangle indicates the docking of SAH (green), the product of the DNA methylation reaction, in the MTase domain surface model (gray). Hydrophobic contacts between the ligand and amino acids residues are in red, with potential hydrogen bonds shown in light blue. Details of interactions between ligands from docking simulation (green sticks) and amino acid residues are shown in (B) SAH, (C) p-coumaric acid, and (D) EGCG. All ligands were overlapped with SAH from the crystallographic model (dark blue sticks). (E) In vitro DNA methylation assay. The absence of BstUI restriction fragments in the methylation reactions containing p-coumaric acid or EGCG indicates no inhibitory effects of methylase M.SssI by these chemical compounds.