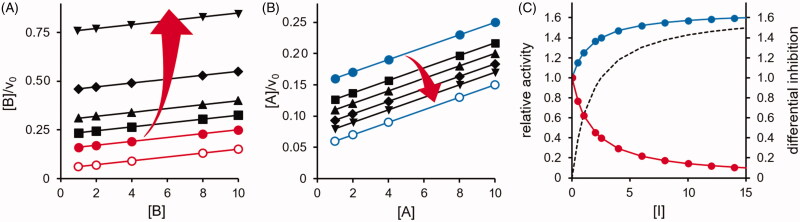
Figure 2.
Predicted effect of a competitive DI on the transformation of two substrates catalysed by a multi-specific enzyme. The kinetic behaviour is described by the model reported in Figure 1 with the two substrates displaying the same KM (i.e. KA = KB = 5 concentration units) and the same kcat (100 time−1 units) and is represented by the Hanes–Wolff plot. Panel A: the transformation of substrate B, which is susceptible to inhibition, is analysed. The red open and closed circles refer to substrate B present alone and in the presence of A at a concentration equal to 2KM, respectively. Symbols ▪, ▲, ♦ and ▼refer to red closed circles in the presence of [I] equal to 1, 2, 3, and 5 times the Ki value, respectively. The Ki value is considered as KM/10. Panel B: the transformation of the substrate A, which is insensitive to direct inhibition, is analysed. Blue open and closed circles refer to substrate A alone and in the presence of B at a concentration equal to 2KM, respectively. Symbols ▪, ▲, ♦ and ▼ refer to blue closed circles in the presence of [I] equal to 0.5, 1, 2, and 5 times the Ki value, respectively. The Ki value is considered as KM/10. In Panels A and B, the increase in [I] is emphasised by the red arrows. In Panel C, the B rate transformation (red curve) and A rate transformation (blue curve) are reported as a function of the inhibitor concentration expressed as Ki fold. The substrate concentrations were considered fixed at the 2KM value and the Ki value is considered as KM/10. The dotted line refers to the differential inhibition.