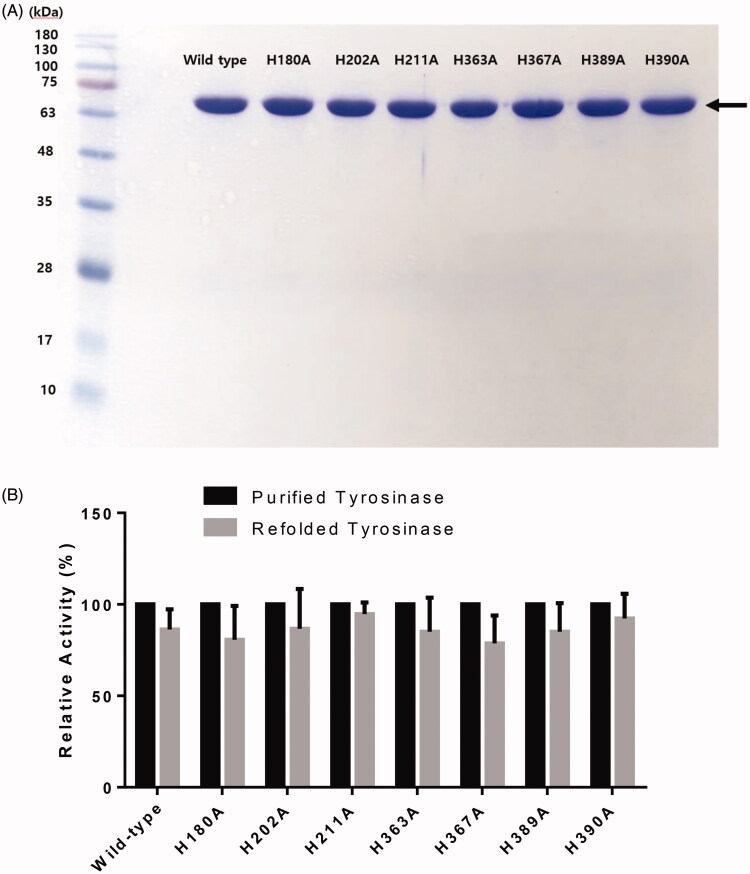
Figure 2.
(A) SDS-PAGE of wild-type human tyrosinase and mutant enzymes after purification through diethylaminoethyl (DEAE)-Sephacel and immobilised metal-affinity chromatography. The gel was stained with Coomassie blue. The arrow indicates the calculated size of 66 kDa, corresponding to human tyrosinase. (B) Confirmation of tyrosinase stability by refolding. The tyrosinase expressed and purified in E. coli BL21 was denatured by adding 8 M urea, and then refolded by gradationally reducing the urea concentration by dialysis. The activity of the refolded tyrosinase was measured and compared to that of the early purified tyrosinase. The values represent the mean ± SD (n = 3).