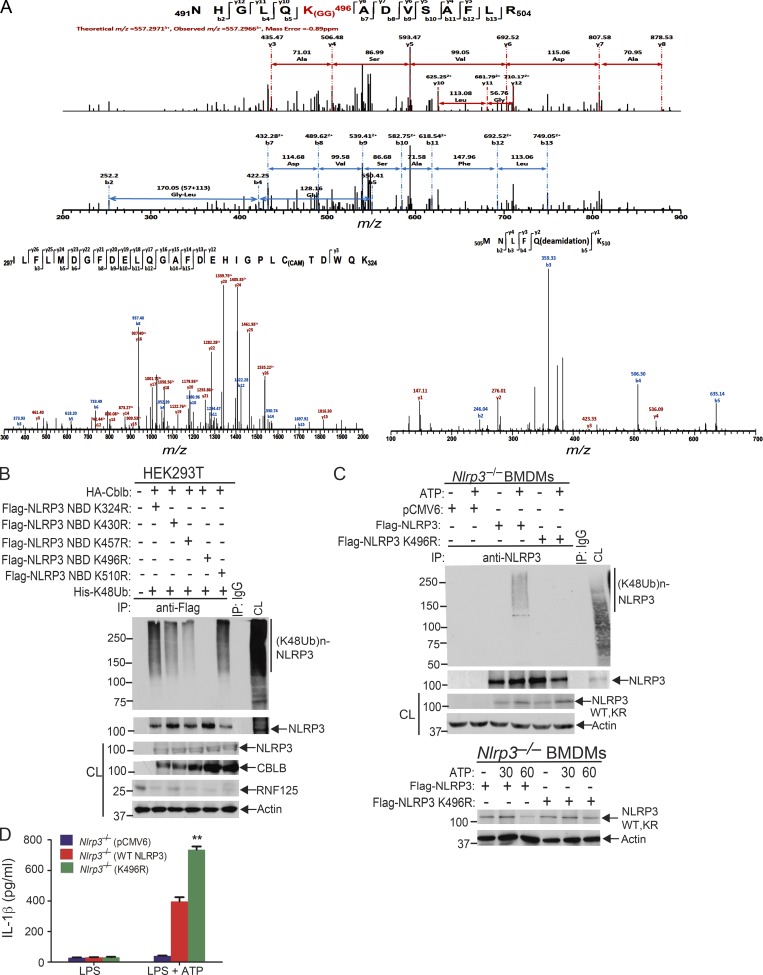
Figure 6.
Lysine 496 is the site for the attachment of K48-linked ubiquitin chains to NLRP3. (A) Mass spectrometric analysis of Flag-tagged NLRP3 showed that K496 [491NHGLQK(GG)496ADVSAFLR504] was ubiquitinated. (B) Anti-His immunoblot analysis of Flag immunoprecipitates (IP) of lysates from HEK293T cells transfected with HA-tagged Cbl-b and His-tagged K48 ubiquitin together with Flag-tagged NLRP3 lysine (K)/arginine (R) mutants (K324R, K430R, K437R, K496R, and K510R). CL, cell lysate. (C) Anti-K48 ubiquitin immunoblot analysis of NLRP3 immunoprecipitates of lysates from Nlrp3−/− BMDMs reconstituted with pCMV6, Flag-tagged NLRP3, or NLRP3 K496R plasmid (upper panel). Anti-NLRP3 and anti-actin immunoblots of lysates from Nlrp3−/− BMDMs reconstituted with Flag-tagged NLRP3 or NLRP3 K496R plasmid, primed with LPS, and stimulated with ATP (lower panel). (D) IL-1β production by BMDMs from Nlrp3−/− mice (n = 3) reconstituted with Flag-tagged NLRP3 or NLRP3 K496R plasmid, primed with LPS, and stimulated with ATP for 30 min. Data are represented as mean ± SD. **, P < 0.01 (Nlrp3−/− BMDMs transfected with WT NLRP3 vs. with NLRP3 K496R); Student’s t test. Data are representative of two independent experiments (A) and representative of three independent experiments (B–D).