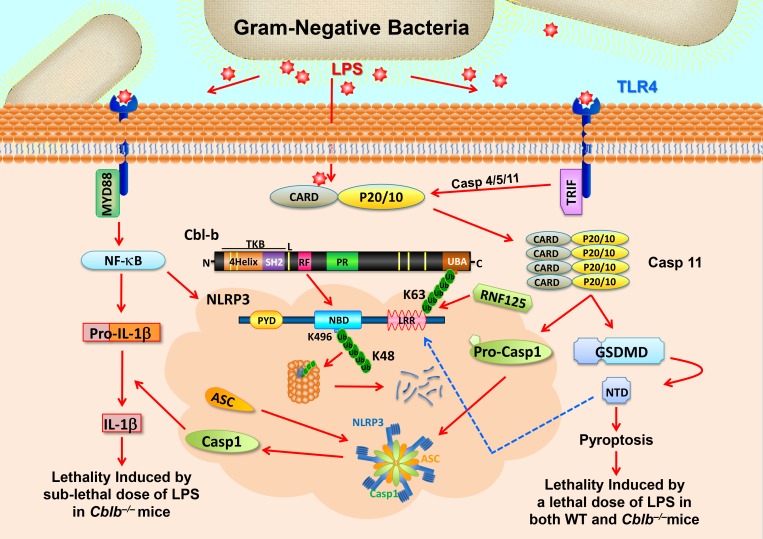
Figure 8.
Schematic model for RNF125 and Cbl-b in LPS-induced endotoxemia. When mice receive a sub-lethal dose of LPS, LPS triggers (1) TLR4 to initiate a MyD88-dependent signaling pathway, which activates NF-κB and induces the expression of NLRP3 and pro–IL-1β and (2) TRIF-dependent pathway that induces the expression of Casp-11. LPS also undergoes endocytosis and binds its cytosolic sensor Casp-11 to induce oligomerizations of Casp-11, which cleaves GSDMD to liberate its N-terminal domain (NTD). NTD may trigger the activation of the NLRP3 inflammasome, leading to the release of IL-1β without inducing pyroptosis. RNF125 targets the NLRP3 LRR domain for K63-linked polyubiquitination. The K63 ubiquitin chains attached to the NLRP3 LRR domain recruit Cbl-b by binding its UBA region. Cbl-b then ubiquitinates NLRP3 at K496 within its NBD and targets NLRP3 to the proteasome for degradation, thus keeping the NLRP3 inflammasome in check. In the absence or inactivation of Cbl-b, mice are highly susceptible to LPS-induced endotoxemia. However, when mice receive a lethal dose of LPS exposure, LPS binds to Casp-11 and mainly triggers Casp-11–mediated pyroptosis via GSDMD. Cbl-b is unable to control this noncanonical inflammasome-induced lethality. CARD, caspase recruitment domain; PYD, pyrin domain; TKB; protein tyrosine kinase-binding; L, linker region; RF, Ring finger; P, peptide; PR, proline-rich region; Toll/IL‐1R domain–containing adaptor‐inducing IFN‐β. Red arrows indicate activation or induction, whereas the blue dashed arrow indicates possible activation.