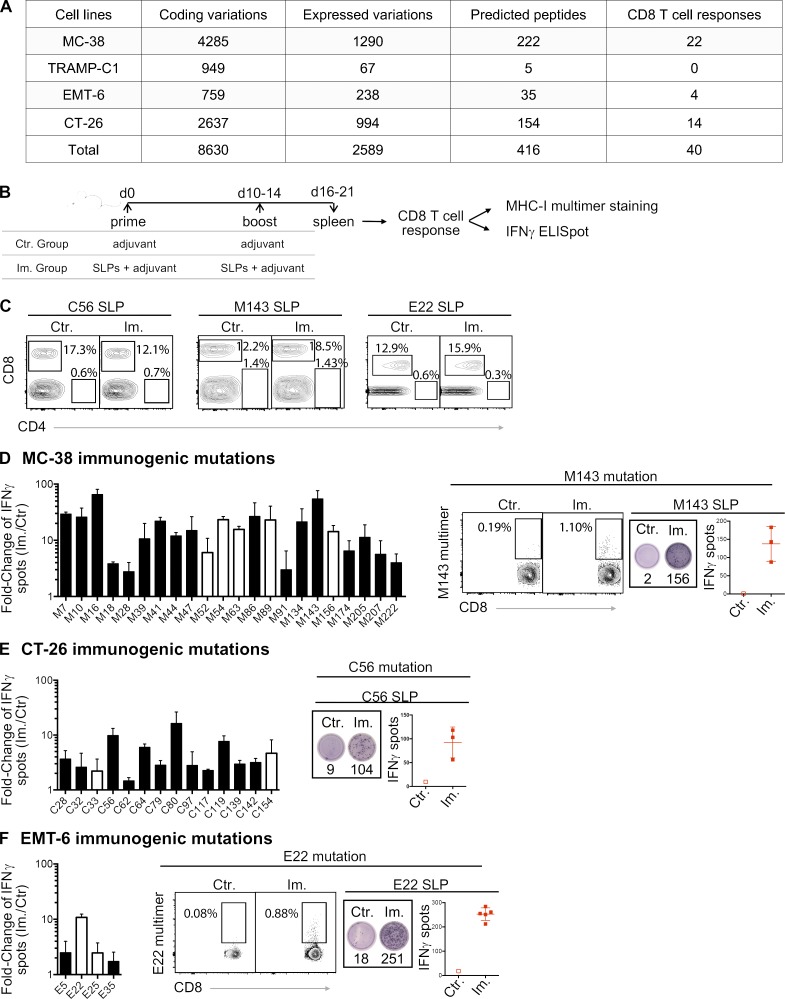
Figure 1.
MHC-I immunogenicity of neoantigen candidates. (A) Number of exome and transcript variations, predicted peptides, and detected CD8 T cell responses for four mouse tumor cell lines (MC-38, TRAMP-C1, EMT-6, and CT-26). (B) Schema depicting the study protocol. Naive mice were immunized with mutant predicted SLPs + adjuvant (Im., immunized; adjuvants: anti-CD40 antibody and poly(I:C)) or adjuvant only (Ctr, control) on day 0 and days 10–14. CD8 T cell responses were assessed in spleen 6–7 d following the last immunization, either by IFN-gamma ELISpot (after CD4 T cell depletion) or MHC-I multimer staining. (C) Representative flow cytometry graphs from purity analysis of CD4 depletion from experiments assessing CD8 T cell responses shown in D–F. Purity of the depletion was ≥98%. (D–F) Representative data (from one experiment, repeated 3–11 independent times) of detected MHC-I immunogenic mutations are shown for each mouse model (D-MC-38, E-CT-26, and F-EMT-6). Anchor (white) and nonanchor (black) mutations are shown for each tumor model. Mean (± SD) fold-change of IFN-gamma spot numbers from CD8 T cells (5 × 105 CD4-depleted splenocytes/well) between control (Ctr., n = 1) and immunized (Im., n = 3–5) mice after overnight stimulation with the mutant predicted SLP (25 µg/ml). Representative data and pictures of mutant-specific MHC-I multimer staining and/or IFN-gamma ELISpot for one mutation are shown for each mouse model. Each image is a representative well from an ELISpot plate.