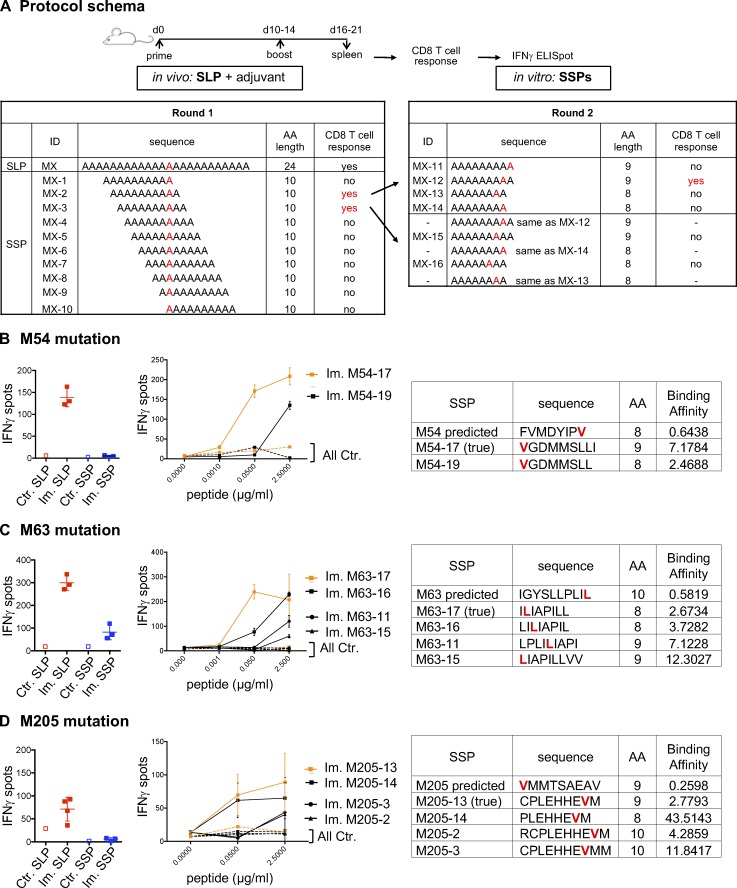
Figure S1.
Immunogenicity of predicted MHC-I optimal mutant epitopes. (A) CD8 T cells (5 × 105 CD4-depleted splenocytes/well) from control (Ctr., n = 1) or immunized (Im., n = 3) mice with mutant SLPs were isolated following the in vivo protocol as shown in Fig. 1 B and in vitro cultured with SLPs or SSPs to assess IFN-gamma release by ELISpot assay. To determine alternative neoepitopes of the poorly or nonimmunogenic predicted neoepitope candidate, IFN-gamma release was measured by neoantigen-specific CD8 T cells generated on SLP vaccines and restimulated in vitro with overlapping 10-, 9-, or 8-mer peptides containing the mutation. (B–D) Representative data of IFN-gamma spots (mean ± SD) from MC-38 mutations (B, M54; C, M63; D, M205) comparing the predicted mutant SLP (25 µg/ml) and optimal SSP (2.5 µg/ml) are shown, as well as the dose response (mean ± SEM to various concentrations) to 10-, 9-, or 8-mer immunogenic alternate optimal mutant peptides. Sequence, length, and predicted BA (H-2Kb for M54 and M63; H-2Db for M205) of each peptide (mutation in red) are shown. Each experiment was independently repeated twice.