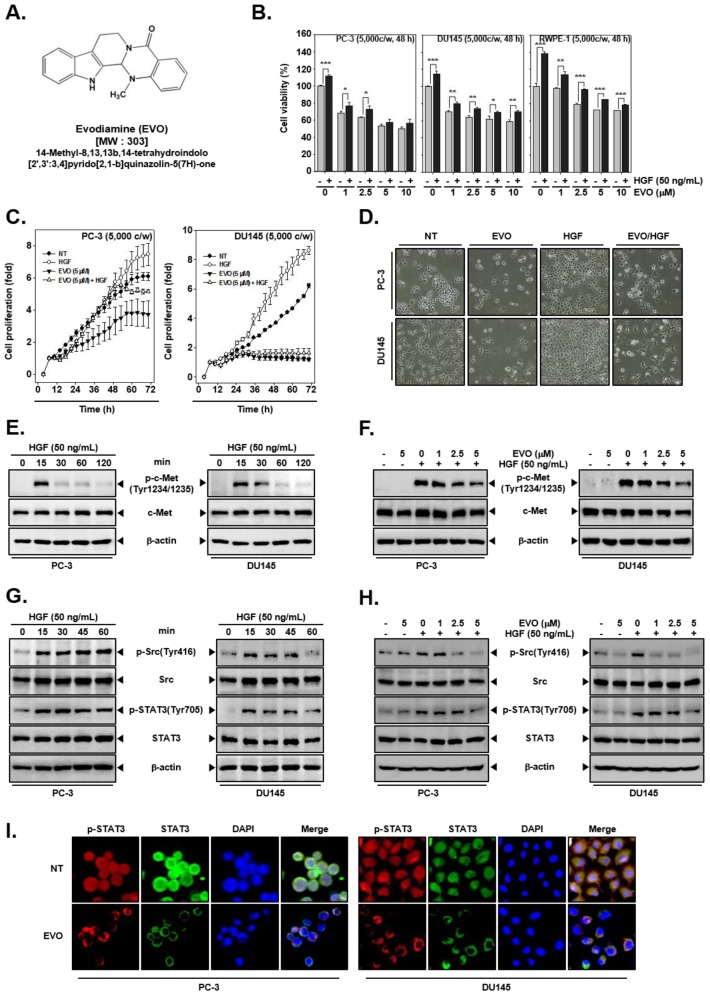
Figure 1.
Inhibition of cell growth by evodiamine (EVO) and hepatocyte growth factor (HGF)-induced c-Met/Src/STAT3 phosphorylation in human prostate cancer cells. (A) Chemical structure of EVO. (B) PC-3 (5 × 103 cells/well), DU145 (5 × 103 cells/well), and normal human prostate (RWPE-1) (5 × 103 cells/well) cells were pre-treated with EVO (0, 1, 2.5, 5, 10 µM) for 1 h and then treated with HGF (50 ng/mL) for a total of 48 h. Cell viability was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. (C) PC-3 (5 × 103 cells/well) and DU145 cells (5 × 103 cells/well) were treated with EVO or HGF. cell proliferation was determined by using real-time cell analysis (RTCA). (D) PC-3 (1 × 105 cells/well) and DU145 cells (5 × 104 cells/well) were treated with EVO or HGF. After 48 h of treatment, cell morphology and density were observed by an optical microscope at a 100x magnification. (E) PC-3 (5 × 105 cells/well) and DU145 cells (5 × 105 cells/well) were treated with HGF (50 ng/mL) for the indicated times to determine when c-Met activation occurred. Whole cell extracts were prepared and immunoblotted with antibodies for phospho-c-Met(Tyr1234/1235) (p-c-MET) and c-Met. (F) PC-3 (5 × 105 cells/well) and DU145 cells (5 × 105 cells/well) were pre-treated with EVO for 4 h and then treated with HGF for 15 min. Whole cell extracts were prepared and immunoblotted with antibodies for phospho-c-Met(Tyr1234/1235) and c-Met. (G) PC-3 (5 × 105 cells/well) and DU145 cells (5 × 105 cells/well) were seeded in six-well plates and incubated overnight in serum-free conditions. Then, they were treated with HGF (50ng/mL) in serum-free conditions for the indicated times to determine when Src and STAT3 were activated. Whole cell extracts were prepared and immunoblotted with antibodies for phospho-c-Src(Tyr416), Src, phospho-STAT3(Tyr705), and STAT3. (H) PC-3 (5 × 105 cells/well) and DU145 cells (5 × 105 cells/well) were seeded in six-well plates and incubated overnight in serum-free conditions. Then, they were pre-treated with EVO for 4 h and treated with HGF for 15 min in serum-free conditions. Whole cell extracts were prepared and immunoblotted with antibodies for phospho-c-Src(Tyr416), Src, phospho-STAT3(Tyr705), and STAT3. (I) PC-3 (3 × 104 cells/well) and DU145 cells (3 × 104 cells/well) were treated with 5 µM EVO for 4 h. The samples were subjected to immunocytochemistry with phospho-STAT3(Tyr705) and STAT3 antibodies. Non-treated (NT) and EVO (−)/HGF (−) indicate control samples treated with medium containing 0.1% DMSO.