Figure 4: BE functionalized with ART inhibit HIV-1 infection.
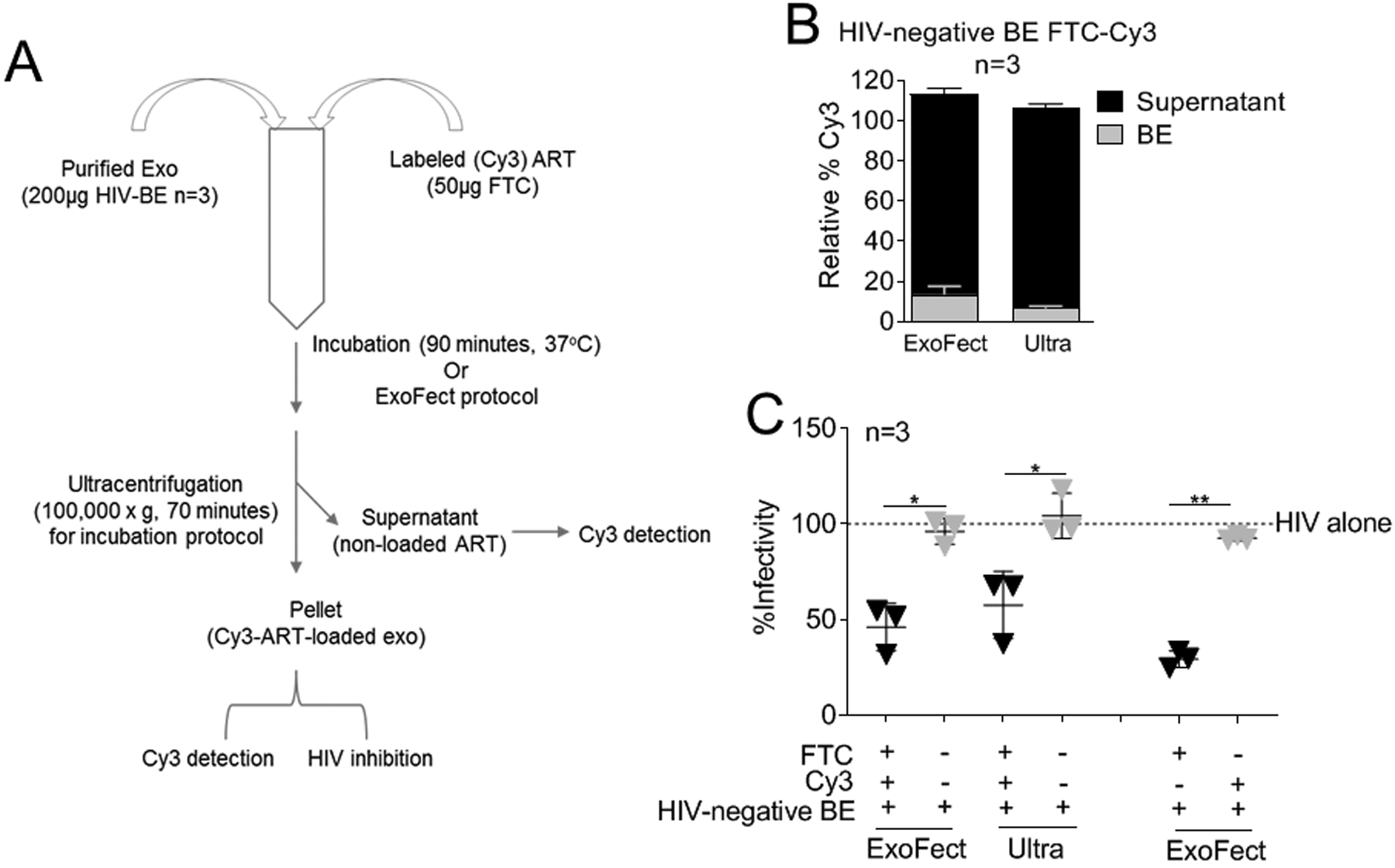
(A) 200 μg BE from HIV-negative donors (n=3) were loaded with 50 μg Cy3 labeled emtricitabine (FTC) by two methods (ExoFect vs. Ultra). (B) Cy3 was detected in the EV pellet and supernatant to determine loading efficiency. Supernatant Cy3 was set at 100% for each donor and loading method. (C) HIV-1 infectivity of 100,000RLU HIV-1 NL4.3 virus in the presence of 100 μg/ml FTC-loaded BE from (A-B), BE-loading controls, or vehicle PBS control on TZM-bl indicator cells for 24 h. TZM-bl infectivity was measured by luciferase reporter activity. Vehicle treated cells are set as reference at 100% (broken line).Statistics was determined by comparing infectivity values of FTC-loaded BE to relevant controls. Significance was determined by student’s t test. *=P<0.05, **=P<0.01, ***=P<0.001. Error bars are SD of biological replicates from the mean of triplicate measurements. ns= not significant.
