Figure 1.
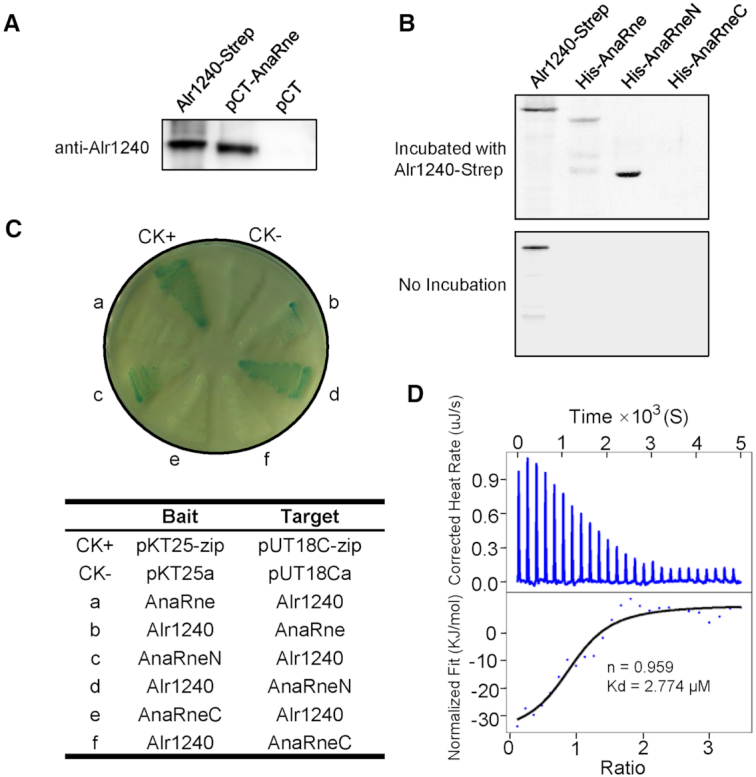
Identifying the interaction between Alr1240 and AnaRne. (A) Alr1240 was co-purified with TwinStrep-tagged AnaRne expressed in Anabaena cells using Strep-Tactin Sepharose. The eluted protein samples from cells carrying the TwinStrep-tagged AnaRne-expressing plasmid (pCT-AnaRne) and the empty vector (pCT) were evaluated using antibodies against Alr1240. The recombinant protein Alr1240-Strep (Alr1240 with a C-terminal Strep tag fusion) served as the positive control. (B) Far-western blot assay showing that Alr1240 interacts with the catalytic domain of AnaRne. Duplicate samples of the full-length, N-terminal half, and C-terminal half of the AnaRne protein, together with recombinant Alr1240-Strep (the positive control), were separated on 10% SDS PAGE gels and transferred onto nitrocellulose membranes. One membrane was incubated with Alr1240-Strep, and the other was not. Subsequently, the signals on both membranes were developed with an antibody against the Strep tag (see Materials and methods for details). (C) Investigating the interaction between Alr1240 and AnaRne by the bacterial adenylate cyclase two-hybrid assay. Escherichia coli BTH101 cells were co-transformed with the indicated two-hybrid plasmid pairs and incubated on plates containing X-gal, IPTG, and the appropriate antibiotics (Kan and Amp) at 30°C for 3 days. Cells co-transformed with pKT25-zip and pUT18C-zip were used as the positive control (CK+), and cells co-transformed with the empty vectors pKT25a and pUT18Ca were used as the negative control (CK–). (D) ITC assay evaluating the interaction between Alr1240 and AnaRneN. The original titration data and integrated heat measurements are shown in the upper and lower plots, respectively.
