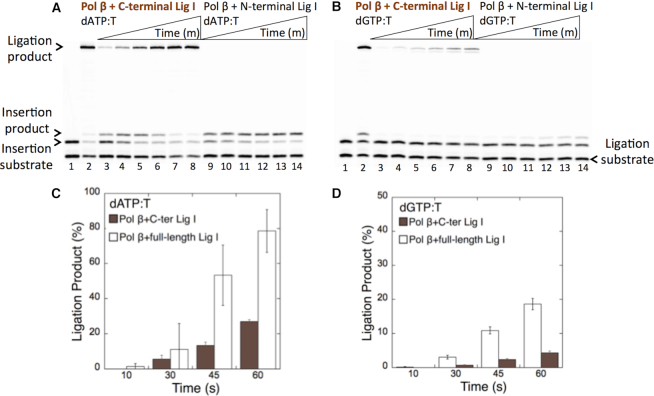
Figure 5.
Effect of DNA ligase I interaction on the ligation of pol β dATP:T and dGTP:T insertion products. In both panels, lane 1 is the negative enzyme control of the one-nucleotide gapped DNA substrate Tcoupled, and lane 2 shows the positive control for the insertion coupled to the ligation product in the reaction including pol β and full-length DNA ligase I. (A) Lanes 3–8 and 9–14 show the pol β insertion coupled to ligation products obtained with the C- and N-terminal domains of DNA ligase I, respectively, in the presence of dATP:T at the time points 30, 45, 60, 120, 180 and 240 s. (B) Lanes 3–8 and 9–14 show the pol β insertion coupled to ligation products obtained with the C- and N-terminal domains of DNA ligase I, respectively, in the presence of dGTP:T at the time points 30, 45, 60, 120, 180 and 240 s. (C andD) The graph shows the time-dependent changes in the ligation products obtained in the coupled reactions including full-length versus the C-terminal domain of DNA ligase I for dATP:T (C) and dGTP:T (D). The data are presented as the averages from three independent experiments ± SDs.