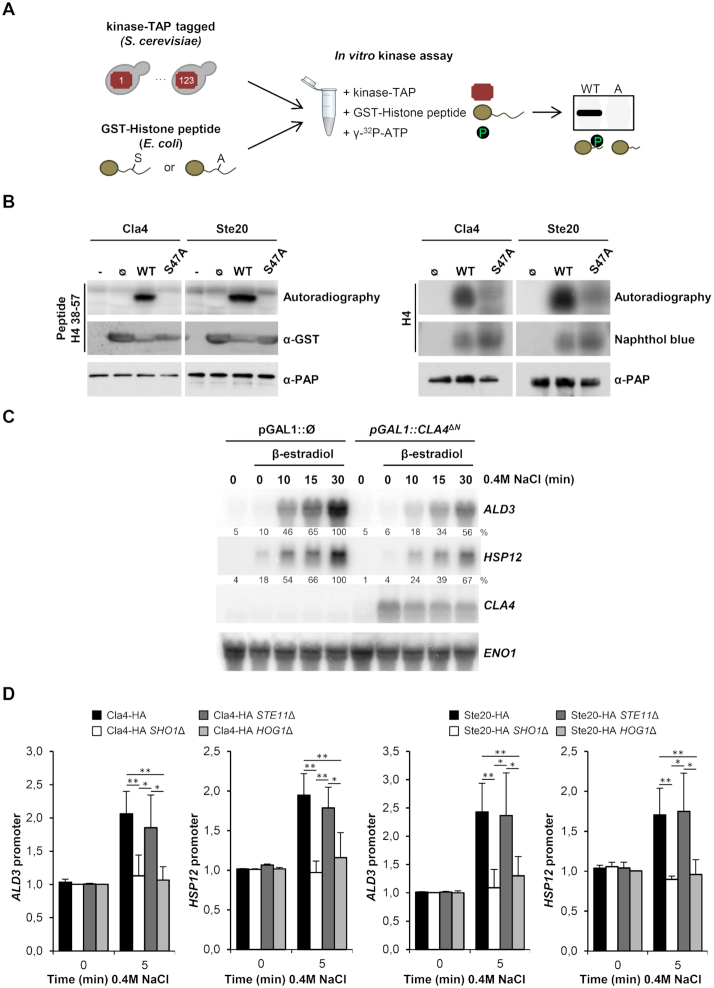
Figure 5.
Cla4 and Ste20 PAKs phosphorylate H4-S47 and regulate osmo-responsive transcription. (A) Experimental strategy to assess the phosphorylation of histone residue H4-S47 in vitro. 123 TAP-tagged kinases were purified from yeast cells and individually assayed with wild-type (WT) and a mutated version (A) of GST-H4 histone peptides purified from E. coli. (B) Cla4 and Ste20 phosphorylate H4-S47 in vitro. Left panel: Fused GST-short peptides (amino acids 38–57) containing the H4-S47 (WT) or the mutated version (S47A) were used as substrates. Empty GST protein (Ø) and no substrate (–) were used as negative controls. Right panel: Purified full-length WT histone H4 or the S47A mutant were used as substrates. Radiolabelled peptides or full-length histones were resolved by SDS-PAGE, transferred to a nylon membrane, and detected by autoradiography. TAP-tagged kinases and GST-tagged histone peptides were detected by western blot. Full-length histones were detected by naphthol Blue. (C) Constitutive activation of Cla4 mimics the osmo-responsive transcriptional defect of H4-S47D mutant cells. mRNA levels of stress-responsive genes (ALD3 and HSP12) upon osmostress (0.4 M NaCl) for the indicated length of time were assessed by northern blot in cells harboring a constitutively activated version of Cla4 (pGAL1::CLA4ΔN) or not (pGAL1:: Ø), which are inducible by β-estradiol addition. RNA levels were quantified as described in Figure 4B. (D) PAKs are recruited to stress-responsive promoters upon osmostress. HA-tagged Cla4 and Ste20 cells were treated with 0.4 M NaCl for the indicated length of time. ChIP against HA was performed to analyze binding to ALD3 and HSP12 promoters. Real-time PCR results are shown as the fold induction relative to the non-treated wild-type strain normalized to a telomere internal control. Data are reported as mean ± SD. *P < 0.05, **P < 0.01.