Background.
Antibody-mediated rejection (AMR) continues to have a deleterious impact on kidney allograft survival. Recent evidence supports use of tocilizumab for treatment of chronic active AMR, but it has not been assessed for treatment of acute active AMR.
Methods.
We performed a single-center, observational study of kidney transplant recipients who received at least 1 dose of tocilizumab in addition to conventional therapies for acute active AMR between October 2016 and October 2018 with follow-up through August 2019.
Results.
Seven patients were included. All 7 patients received tocilizumab 8 mg/kg (max dose, 800 mg) monthly. We noted a 50% or greater reduction in immunodominant donor-specific antibodies in 4 of 6 patients. Renal function improved or stabilized in all patients throughout the duration of therapy. One patient developed cytomegalovirus esophagitis and 1 had a potential hypersensitivity reaction. In the extended follow-up, 1 patient had mixed rejection and 2 patients had T-cell–mediated rejection, which occurred 6 to 24 mo after completion of therapy.
Conclusions.
Tocilizumab may be considered as an addition to conventional therapies for treatment of acute active AMR. More studies are needed to determine which patients may benefit from therapy and to examine the appropriate duration of treatment.
Antibody-mediated rejection (AMR) continues to have a deleterious impact on kidney allograft survival.1 Current evidence for treatment of acute active AMR is limited, but treatment recommendations were recently released. The 2019 Expert Consensus from the Transplantation Society Working Group described the combination of plasmapheresis (PP), IVIG, and steroids as the standard of care for most cases of acute active AMR and highlighted that adjunctive therapies may also be considered depending on the clinical situation. This group also indicated that new agents and adequately powered clinical trials are urgently needed to improve patient outcomes.2
Recently, there has been interest in targeting interleukin 6 (IL-6). Interleukin 6 mediates various inflammatory and immunomodulatory pathways. Notably, in kidney transplantation, it is critical for expansion and activation of T cells and B cells. Evidence suggests IL-6 controls proliferation and survival of T-cells, including Tfh and Th17 cells, and also inhibits Treg cell function. Furthermore, IL-6 controls progression of naïve B cells and plasmablasts into mature plasma cells.3
Tocilizumab, an IL-6 receptor antagonist, has been evaluated in the treatment of chronic, active AMR (cAMR) with positive donor-specific antibodies (DSAs) and transplant glomerulopathy resistant to traditional treatment with IVIG and rituximab with and without PP. The study showed a stabilization of renal function over 2 y, and a significant reduction of glomerulitis, peritubular capillaritis, C4d deposition, and DSAs. However, no decrease in transplant glomerulopathy was observed.4 Given these findings, there is interest in using tocilizumab for acute active AMR.
Here, we report 7 cases that received tocilizumab for treatment of acute active AMR. Tocilizumab was used in addition to conventional therapies.
MATERIALS AND METHODS
We performed a retrospective chart review of kidney transplant recipients at Barnes-Jewish Hospital/Washington University Transplant Center who received at least 1 dose of tocilizumab for treatment of acute active AMR between October 2016 and October 2018. We excluded all patients with chronic glomerulopathy (cG) >1. Patients were followed through August 2019. Information about baseline demographics, pertinent comorbidities, and transplant characteristics was recorded. All patients had a renal allograft biopsy performed at the time of rejection diagnosis. DSA testing was also performed at this time and during follow-up by single-antigen bead assay (One Lambda, West Hills, CA). All serum samples were pretreated with ethylenediaminetetraacetic acid.5 A mean fluorescence intensity (MFI) cutoff value of 1000 was used to classify positive DSA, and an MFI >2000 correlates with a positive flow cytometric crossmatch (FCXM) at our center.6 For each patient, the immunodominant DSA was defined as the specificity with the highest MFI among all donor-specific reactivities.
RESULTS
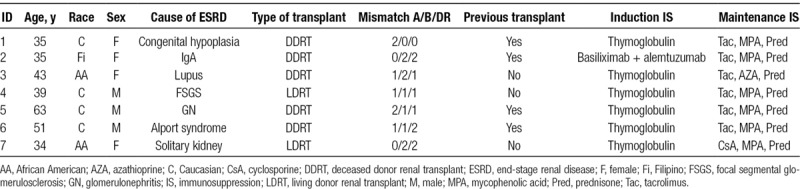
All patients received induction with lymphocyte-depleting agents at the time of kidney transplantation, and all patients were maintained on triple immunosuppression with calcineurin inhibitor, antimetabolite, and prednisone at the time of AMR diagnosis. Baseline characteristics are summarized in Table 1.
TABLE 1.
Baseline characteristics
All patients received tocilizumab in addition to conventional treatments for AMR. Tocilizumab was dosed at 8 mg/kg (max dose, 800 mg) IV monthly. Treatment duration was determined based on patient-specific factors, with treatment duration ranging from 1 to 6 doses. Median duration of treatment was 4 mo.
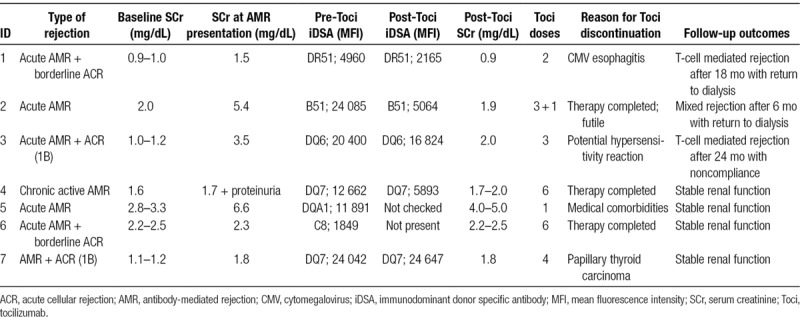
Table 2 shows the Banff scoring system in all biopsies before use of tocilizumab. The majority of patients had high-level DSAs at the time of biopsy. There was a 50% or greater reduction in immunodominant DSA in 4 of 6 patients who had repeat DSA testing following use of tocilizumab. Furthermore, renal function improved or stabilized in all patients (Table 3).
TABLE 2.
Pathology: Banff scoring of kidney transplant biopsies before use of tocilizumab
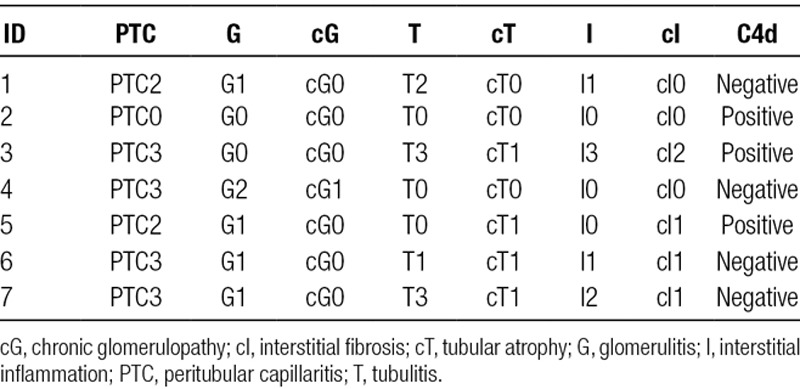
TABLE 3.
Clinical and laboratory characteristics
All cases are discussed in detail below.
Case 1
Thirty-five-year-old Caucasian woman with end-stage renal disease (ESRD) secondary to congenital renal hypoplasia. She underwent living donor renal transplant (LDRT) in 1995, which failed in 1997 after excessive bleeding related to a biopsy. She then underwent deceased donor renal transplant (DDRT) in August 1997, which failed in November 1998 secondary to acute rejection. She received another DDRT in March 2017. HLA mismatch was 2A/0B/0DR. cPRA was 100%, with positive T-cell and B-cell FCXM. DSA screen was negative. She received induction with Thymoglobulin 5 mg/kg and a single dose of rituximab. She was discharged with serum creatinine (SCr) of 0.87 mg/dL.
Two mo after transplant, she developed AKI with SCr of 1.5 mg/dL. Allograft biopsy revealed acute active AMR and borderline acute cellular rejection (ACR). C4d staining was negative. She had new DSA to DR51 (MFI: 4960) and A11 (MFI: 2142). She received a prednisone burst and tocilizumab. Upon discharge, the plan was to continue monthly tocilizumab for 6 mo.
Before the third dose of tocilizumab, the patient reported persistent gastric reflux. Esophagogastroduodenoscopy showed cytomegalovirus (CMV) esophagitis. Valganciclovir was initiated, and tocilizumab was discontinued. When tocilizumab therapy concluded, her renal function had returned to baseline. DSA screen showed a reduction in the level of DSA to DR51 (MFI: 2165).
Her renal function remained stable for nearly 18 mo after discontinuation of tocilizumab until she was readmitted with SCr >10 mg/dL in December 2018. Allograft biopsy revealed ACR, Banff IIA. DSA screen showed DSA to DR51 (MFI: 1264). She received bolus steroids and Thymoglobulin, but her renal function did not recover and she was placed on hemodialysis.
Case 2
Thirty-five-year-old Filipino woman with ESRD secondary to biopsy-proven IgA nephropathy. She underwent LDRT from her husband in 2007, which failed in 2009 secondary to chronic rejection. She then underwent DDRT in October 2016. HLA mismatch was 0A/2B/2DR. cPRA was 100%, with negative T-cell and B-cell FCXM. DSA screen was negative. She received induction with basiliximab followed by alemtuzumab. Her postoperative course was complicated by slow graft function, and she was discharged on POD 8 with SCr of 2 mg/dL.
She was readmitted 4 d later with SCr of 5.4 mg/dL. She had new DSA against B51 (MFI: 24 085), B53 (MFI: 20 801), DQ2 (MFI: 14 965), DR13 (MFI: 9857), C6 (MFI: 6603), and DR7 (MFI: 4541). Allograft biopsy revealed acute active AMR with thrombotic microangiopathy. C4d staining was diffusely positive. She was initially treated with bolus steroids, PP, and IVIG. She also received eculizumab and rituximab. Following these therapies, a repeat allograft biopsy was performed. There was mildly increased capillary loop thickness and acute tubular necrosis, but no light microscopy evidence of AMR. Nevertheless, based on previous biopsy findings, diffusely positive C4d staining, and presence of DSAs, she was deemed to have acute AMR. At this time, tocilizumab was initiated. The patient’s SCr decreased, and she was discharged with SCr of 3.0 mg/dL. Upon discharge, the plan was to continue twice weekly PP, monthly IVIG, and monthly tocilizumab.
PP was discontinued after 2 mo because of line-associated bacteremia. Both IVIG and tocilizumab were discontinued after 3 mo. At the completion of therapy, her SCr had returned to baseline of ~2.0 mg/dL, and her DSAs had decreased significantly. DSA to B51 decreased from 24 085 to 5064. DSA to B53 decreased from 20 801 to 2719.
The patient did well for 6 mo until she was admitted to a different medical center with SCr of ~6.0 mg/dL in July 2017. Allograft biopsy showed ACR, Banff IB, with moderate glomerulitis and minimal peritubular capillaritis. She received bolus steroids and Atgam, but her SCr remained elevated at 5–6 mg/dL. She received 1 additional dose of tocilizumab in August 2017 before it was discontinued because of low likelihood of clinical response. She was initiated on hemodialysis in November 2017.
Case 3
Forty-three-year-old African American woman with ESRD secondary to lupus nephritis. She underwent DDRT in November 2009. HLA mismatch was 1A/2B/1DR. T-cell and B-cell FCXM were negative. Induction was Thymoglobulin 5 mg/kg. Baseline SCr was 1.0–1.2 mg/dL.
In June 2017, she developed AKI with SCr of 3.5 mg/dL. Allograft biopsy revealed ACR, Banff IB, with increased plasma cells; acute active AMR; focal global glomerulosclerosis 9 of 32 (28%); and moderate interstitial fibrosis. C4d staining was focally positive. She had DSA to DQ6 (MFI: 20 400), DR51 (MFI: 17 524), C7 (MFI: 2402), and B53 (MFI: 1528). She did not undergo PP because of difficult access. Instead, she received bolus steroids, Thymoglobulin, IVIG, and tocilizumab. Her SCr decreased, and she was discharged with SCr of 2.1 mg/dL. Upon discharge, the plan was to continue monthly tocilizumab for 6 mo.
After the third dose of tocilizumab, she developed dyspnea. Diphenhydramine and acetaminophen were administered. She subsequently became unresponsive. Epinephrine was administered, and cardiopulmonary resuscitation was commenced. She was revived without requiring intubation. The syncope was attributed to an adverse reaction from tocilizumab or intravenous diphenhydramine. Because of the uncertainty of this reaction, subsequent tocilizumab infusions were discontinued. Her SCr stabilized around 2.0 mg/dL.
Approximately 24 mo later, she presented with AKI with SCr of 5.2 mg/dL in the setting of medication noncompliance. Allograft biopsy showed ACR, Banff IB. C4d staining was negative. She had DSA to DQ6 (MFI: 10 414) and DR51 (MFI: 16 746). Bolus steroids and Thymoglobulin were administered, and her SCr decreased to 3.5 mg/dL.
Case 4
Thirty-nine-year-old Caucasian man with ESRD secondary to biopsy-proven focal segmental glomerulosclerosis (FSGS). He underwent LDRT from his mother in July 2015. It was a 1-haplotype match. T-cell and B-cell FCXM were negative. Induction was Thymoglobulin 5 mg/kg. Baseline SCr was 1.6 mg/dL.
In January 2018, he developed new onset proteinuria of 1.8 g. Allograft biopsy showed PTC3, g2, and cG1. C4d staining was negative. He had new DSA against DQ7 (MFI: 12 662), so he was considered to have AMR. He received bolus steroids, PP, IVIG, bortezomib, rituximab, and tocilizumab. Upon discharge, the plan was to continue monthly tocilizumab for 6 mo.
He completed 6 mo of tocilizumab. His SCr was stable at 1.7–2.0 mg/dL throughout treatment with proteinuria <0.5 g. An interim biopsy in March 2018 showed FSGS, 69% global glomerulosclerosis, and severe interstitial fibrosis. Approximately 1 mo after treatment concluded, proteinuria increased to ~1.5 g and then reached 5.4 g in December 2018. A repeat biopsy showed PTC0, g2, cG1, and FSGS (8/20). C4d staining was negative. ACTH gel was initiated with rising proteinuria but was subsequently discontinued because of intolerance. Thus, lisinopril was maximized. SCr has remained stable around 2 mg/dL and proteinuria has been stable around 1 g for the past 10 mo.
Case 5
Sixty-three-year-old Caucasian man with ESRD secondary to unspecified glomerulonephritis. He underwent LDRT from his sister in 1991, who was lost in 2017 because of chronic allograft nephropathy. He then underwent DDRT in February 2018. HLA mismatch was 2A/1B/1DR. cPRA was 74%, with negative cytotoxicity crossmatch and positive B-cell FCXM. He had DSA to DQA1*02:01 (MFI: 15 877), C5 (MFI: 3076), and DR7 (MFI: 2194). Induction was Thymoglobulin 5 mg/kg. He also received PP, rituximab, and bortezomib. His postoperative course was complicated by delayed graft function. Allograft biopsy performed on POD 13 showed acute tubular injury and mild glomerulitis. C4d staining was negative. His SCr continued to decrease with baseline SCr noted to be 2.8–3.3 mg/dL.
Two months after transplant, he developed AKI with SCr of 6.6 mg/dL and worsening proteinuria. Allograft biopsy revealed active AMR. Minimal C4d staining in peritubular capillaries (<10%) was observed. He had persistent DSA to DQA1*02:01 (MFI: 11 891). He received bolus steroids, PP, IVIG, rituximab, and tocilizumab. His SCr decreased, and he was discharged with SCr of 4.4 mg/dL.
Within 2 wk of discharge, he was readmitted with hematochezia. Notably, he was taking warfarin for atrial fibrillation. Colonoscopy revealed several sites with ulceration and exposed visible vessel throughout the distal transverse, descending, sigmoid colon, and rectum. Hospital course was complicated by pancytopenia, aspiration pneumonia, and abdominal abscess. Further treatments for rejection were not pursued because of his medical comorbidities. His SCr has remained stable around 3.5–4.0 mg/dL for the past 18 mo.
Case 6
Fifty-one-year-old Caucasian man with ESRD secondary to Alport syndrome. He underwent DDRT in 2007, which was complicated by rejection and return to dialysis in 2014. He received a second DDRT in June 2017. HLA mismatch was 1A/1B/2DR. cPRA was 87%, with negative T-cell and B-cell FCXM. He had DSA to C8 (MFI: 2356). Induction was Thymoglobulin 5 mg/kg. He was discharged with SCr of 1.9 mg/dL.
Two months after transplant, he developed AKI with SCr of 4.8 mg/dL. Allograft biopsy revealed acute active AMR. C4d staining was negative. He had DSA to C8 (MFI: 3698), DR14 (MFI: 1032), and DR15 (MFI: 1112). He received bolus steroids, PP, and study medication (C1 esterase inhibitor versus placebo). His SCr decreased, and he was discharged with SCr of 2.7 mg/dL.
Approximately 6 mo after this episode of AMR, repeat allograft biopsy showed acute active AMR and borderline ACR. C4d staining was negative. He had DSA to C8 (MFI: 1849), DR14 (MFI: 1090), and DR15 (MFI: 1193). He received bolus steroids, PP, IVIG, rituximab, and tocilizumab.
He completed 6 mo of tocilizumab as planned. Upon completion of therapy, DSA screen was negative. His SCr has remained stable at 2.2–2.5 mg/dL for the past 15 mo.
Case 7
Thirty-four-year-old African American woman with ESRD secondary to solitary kidney and proteinuria. She underwent LDRT from a friend in June 2012. HLA mismatch was 0A/2B/2DR. T-cell and B-cell FCXM were negative. Induction was Thymoglobulin 5 mg/kg. Baseline SCr was 1.1–1.2 mg/dL.
In April 2018, she developed AKI with SCr of 1.8 mg/dL in the setting of medication noncompliance. Allograft biopsy revealed active AMR; ACR, Banff IB; and moderate IFTA. C4d staining was negative. She had DSA to DQ7 (MFI: 23 344). She received bolus steroids, PP, IVIG, Thymoglobulin, and rituximab. Despite these therapies, her DSAs remained unchanged (MFI: 24 042), and her SCr remained elevated around 1.8 mg/dL.
In June 2018, tocilizumab was initiated. Around this same time, a mass was found on the right anterior neck. Biopsy revealed papillary thyroid carcinoma, and she underwent total thyroidectomy. Given the newly diagnosed cancer, tocilizumab and mycophenolic acid were discontinued. She ultimately received 4 doses of tocilizumab. Her DSAs remained unchanged and SCr remained stable for the past 12 mo.
DISCUSSION
AMR can have detrimental effects on allograft survival and quality of life for kidney transplant recipients. PP, IVIG, and steroids have become the standard of care, yet these strategies have not proven to be adequate for treatment of AMR.2
Tocilizumab has previously been studied in the treatment of cAMR. In that study of 36 patients who failed standard therapy for cAMR, significant reductions in immunodominant DSAs and stabilization of renal function were seen at 2 y. Furthermore, tocilizumab-treated patients demonstrated graft survival and patient survival rates of 80% and 91% at 6 y, respectively.4
Our study provides novel insight on use of tocilizumab in patients presenting with acute active AMR. Patients with acute active AMR have an increased risk of chronic AMR and graft loss.7 Thus, treatment strategies aimed at removing circulating DSAs and/or reducing DSA production may be beneficial in this population to delay or prevent progression. Conventional therapy with PP followed by IVIG has been shown to reduce DSA levels by 15%–35% depending on HLA DSA specificity and number of PP sessions.8 Therefore, we deemed a 50% reduction in DSA levels to be clinically meaningful. In the present study, a 50% or greater reduction in immunodominant DSA was observed in 4 of 6 patients, and DSA stabilized in all other patients. Furthermore, renal function improved or stabilized in all patients during therapy.
In terms of efficacy, 1 patient experienced mixed rejection 6 mo after completion of tocilizumab, while 2 others experienced ACR at 18 and 24 mo after medication discontinuation. Two of these patients ultimately returned to dialysis secondary to these recurrent rejection episodes. It should be noted that all patients who experienced rejection received ≤3 doses of tocilizumab with their initial course. Rebound IL-6 activity after stopping tocilizumab has been suggested in studies using it for desensitization, although the clinical significance remains unknown.9 Our early experience suggests that ongoing treatment may be warranted with tocilizumab.
Adverse events were noted in our case series, although these events could not be attributed directly to tocilizumab since patients also received other conventional therapies. In the study by Choi et al, infectious adverse events were reported in 13 of 36 patients, but all infectious events resolved with directed treatment and without the need to discontinue tocilizumab.4 In our case series, 1 patient developed CMV esophagitis, which was successfully treated with oral valganciclovir. Notably, this patient had a history of CMV viremia before use of tocilizumab. One patient also experienced a potential hypersensitivity reaction. Although uncommon, hypersensitivity reactions have been reported in association with tocilizumab.10
Several limitations of our study require consideration. The study is limited by the small cohort size, the heterogeneity of the patient population, and the absence of a comparator group. Furthermore, treatment for AMR at our institution is not managed by standardized protocols. Given that all patients received other conventional therapies, it was difficult to assess the contribution of tocilizumab to overall efficacy. Additionally, although a reduction in DSA levels was noted for several patients, some DSAs remained strong enough to cause a positive FCXM. Our study is also limited by the lack of protocol biopsies following use of tocilizumab, although several patients had a repeat biopsy performed when they presented with additional episodes of AKI.
In summary, our study provides novel insight into the use of tocilizumab for treatment of acute active AMR. Further studies are needed to better define the role of tocilizumab in this context. Our early experience suggests clinicians should give careful consideration to longer durations of therapy.
Footnotes
Published online 13 March, 2020.
The authors declare no funding or conflicts of interest.
A.A.P., C.L., and T.A. performed the study design, data analysis, interpretation, and writing of the article. K.V., D.C.B., H.M., and A.F.M. performed the study design, interpretation, and writing of the article.
REFERENCES
- 1.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 2.Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 Expert Consensus from the Transplantation Society Working Group. Transplantation 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan SC, Choi J, Kim I, et al. Interleukin-6, A cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL-6 receptor blockade. Transplantation. 2017;101:32–44. [DOI] [PubMed] [Google Scholar]
- 4.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Pang S, Phelan D, et al. Quantitative evaluation of the impact of ethylenediaminetetraacetic acid pretreatment on single-antigen bead assay. Transplant Direct. 2017;3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liwski RS, Greenshields AL, Conrad DM, et al. Rapid optimized flow cytometric crossmatch (FCXM) assays: the Halifax and Halifaster protocols. Hum Immunol. 2018;79:28–38. [DOI] [PubMed] [Google Scholar]
- 7.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada C, Ramon DS, Cascalho M, et al. Efficacy of plasmapheresis on donor-specific antibody reduction by HLA specificity in post-kidney transplant recipients. Transfusion. 2015;55:727–35; quiz 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation. 2015;99:2356–2363. [DOI] [PubMed] [Google Scholar]
- 10.Actemra (tocilizumab) [prescribing information]. November 2019San Francisco, CA: Genentech, Inc.; [Google Scholar]


