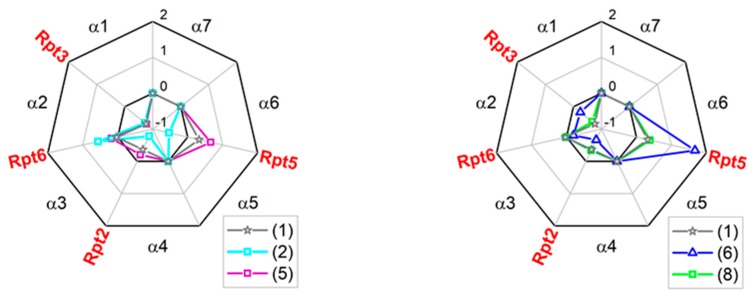
Figure 7.
TAT1 derivatives competed with selected C-terminal tails of Rpt (Regulatory Particle ATPases) subunits for binding to specific α face pockets. The scores presented in radar plots were calculated as ((relative activation by the [x] and Rpt tail)-(theoretical sum of activation by [x] and Rpt tail))/(theoretical sum of activation by [x] and Rpt tail), where [x] represent any TAT peptide. Score = 1 indicates a pure additive effect of Rpt and TAT peptides, score < 1 suggests competition, score > 1 hints at synergy. (5) competed fairly specifically with Rpt3 tail, expected to dock in the α1/α2 pocket, as evident from the strongly negative score. (1) and (8) added a weakly negative score for the Rpt2 tail to the presumed major competitor Rpt3. In turn, Rpt2 tail was a major competitor for (6). The poor activator (2) was the least specific ligand of the α face, competing with Rpt2, Rpt3, and Rpt5 tails. The positive scores for combinations of selected compounds with Rpt5 or Rpt6 tails indicated a presumed synergy in activation of the core. Average scores derived from three to six experiments are presented. The competition effects were statistically significant (at least p < 0.05) for Rpt3 peptide and (1) and (8), as well as for Rpt2 peptide and (6).