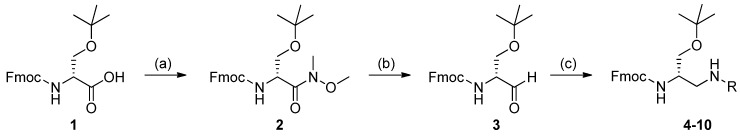
Scheme 2.
Synthesis of Fmoc-2,3-diaminopropanols 4–10. Experimental conditions: preparation of the Weinreb–Nahm amide 2, (a) 1-hydroxybenzotriazole (HOBt) monohydrate/N,N′-diisopropylcarbodiimide (DIC)/1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 2 h, room temperature (r.t.); then, N,O-dimethylhydroxylamine hydrochloride/DIEA/DCM, overnight, r.t. (94% yield); preparation of the Garner-like aldehyde 3, (b) LiAlH4/THF, 12 min, r.t. (92% yield); reductive amination, (c) amine or arylsulfonamide/Ti(OiPr)4/EtOH, 10 min, r.t., then NaBH3CN, overnight, r.t. (82%–92%, Table 1).