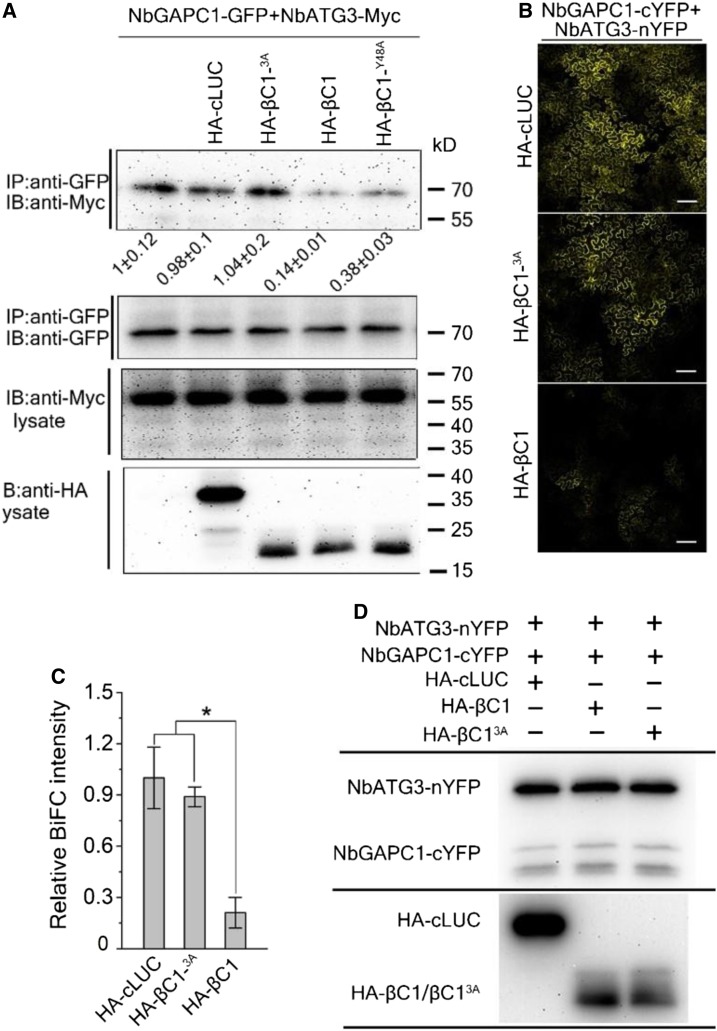
Figure 4.
βC1-GAPCs Interaction Is Important for Disrupting the ATG3-GAPCs Interaction in Plants.
(A) Co-IP assay shows that βC1, but not its mutants βC13A and βC1Y48A, interferes with the interaction between NbGAPCs and NbATG3. Total protein extracts were immunoprecipitated with anti-GFP beads and monitored by immunoblotting (IB) using an anti-Myc antibody. Protein levels were assessed using anti-GFP, anti-Myc, or anti-HA antibodies. Values represent means ± se from three independent experiments.
(B) Confocal image of BiFC assays show that βC1, but not βC13A, interferes with the interaction between NbGAPCs and NbATG3. Photographs were taken at 48 hpi. Scale bar = 200 μm.
(C) BiFC intensity (means ± SEM, n = 4) was quantified by YFP fluorescence. Relative BiFC intensity was normalized to the control. The raw data were analyzed by two-sample t tests to show the significance level of P ≤ 0.05 (*).
(D) Immunoblot analyses of BiFC construct combinations from the same experiments as in (B). The protein level of cYFP-NbGAPC1 and NbATG3-nYFP were assessed with the polyclonal GFP antibody (Huaxin Bochuang). The PVDF membrane was stained with Ponceaux to visualize the large subunit of ribulose-1,5-bisphosphate as a loading control.