Figure 3.
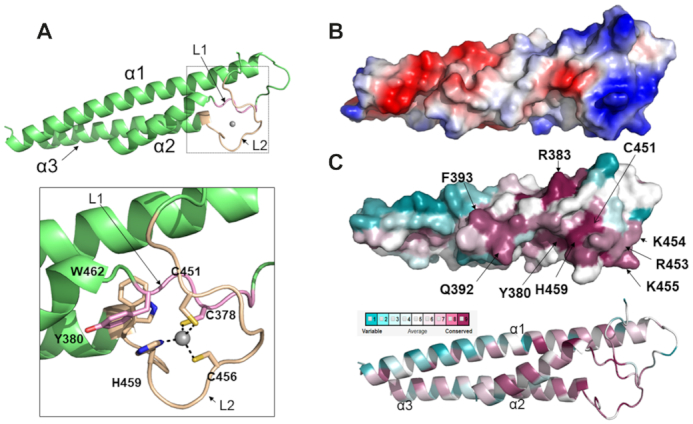
The structure of CtCfp1 RID domain unravels a mono-valent zinc finger. (A) CtCfp1 RID domain is formed by a three-helix bundle in which the secondary structure, the zinc binding motifs (I and II) and the zinc atom are rendered in green, light pink, beige and grey, respectively. The inlet shows the residues found in motifs I and II. Zn is coordinated with four ligands. C378 is located in motif I and rendered in light pink. C456, C451 and H459 are found in motif II and are colored in beige. (B) Electrostatic potential surface of CtCfp1 RID. Electrostatic surface of Cfp1 RID (blue and red denote positively and negatively charged regions, respectively). (C) Conservation of surface residues of Cfp1 RID. Sequence conservation mapped onto Cfp1 are as follows: gradient: dark pink (conserved) to blue (variable).
