Table 2.
ITC binding data for interaction of E. coli SSB C-terminal P15 and RecO in buffer BTP
| pH | [NaCl] (mM) | Temp. | Stoichiometry (N) | K (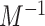 ) ) |
ΔH (kcal/mol) | ΔG° (kcal/mol) | TΔS° (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 8 | 50 | 25°C | 1 (fixed) | (1.2 ± 0.3) × 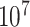
|
−5.2 ± 0.1 | −9.7 ± 0.2 | 4.5 ± 0.2 |
| 8 | 200 | (3.1 ± 0.3) × 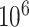
|
−3.9 ± 0.1 | −8.9 ± 0.1 | 5.0 ± 0.1 | ||
| 7 | 200 | 15°C | (1.9 ± 0.5) × 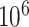
|
−2.4 ± 0.2 | −8.3 ± 0.2 | 5.9 ± 0.3 | |
| 25°C | (1.1 ± 0.1) × 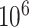
|
−6.0 ± 0.2 | −8.1 ± 0.2 | 2.0 ± 0.3 | |||
| 37°C | (4.5 ± 1.1) × 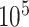
|
−7.8 ± 0.8 | −8.1 ± 0.1 | 0.13 ± 0.1 |
K = observed association equilibrium constant, ΔH = enthalpy change.
