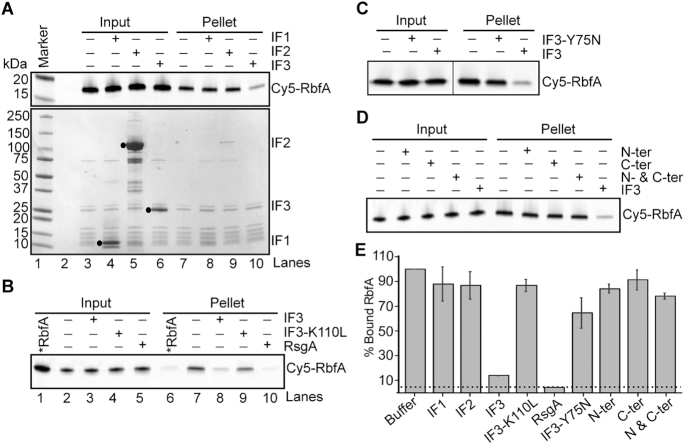
Figure 1.
Full-length IF3 is required for RbfA release from 30S ribosomes. (A) Results of pelleting assay showing the release of Cy5-RbfA from 30S•Cy5-RbfA complexes in the presence of initiation factors. Only IF3 was able to displace RbfA from 30S subunits (lane 10). A complex of mature 30S (200 nM) and Cy5-RbfA (400 nM) was formed and unbound Cy5-RbfA was removed by filtration (input) before complexes were challenged with initiation factors (4 μM). Proteins were resolved by 4–20% SDS PAGE. Top panel, Cy5-RbfA fluorescence; bottom panel, Coomassie stain. Initiation factors (input) are indicated with black dots. (B) Pelleting assay showing that a non-binding IF3 mutant (IF3-K110L) cannot release of RbfA from 30S subunits (lane 9). RsgA (500 nM) and GTP (5 μM) was used as a positive control (lane 10). *RbfA (lanes 1 and 6); Cy5-RbfA only. (C) Results of pelleting assay showing that a mutation in the linker region of IF3 (IF3-Y75N) reduces Cy5-RbfA release. (D) Separated N- and C-terminal domains of IF3 were used alone or in combination. (E) Fraction of bound Cy5-RbfA in pellets from panels (A)–(D). Bars indicate mean and s.d., n = 3 independent trials. Dotted line indicates ∼5% Cy5-RbfA background in reactions lacking 30S subunits.