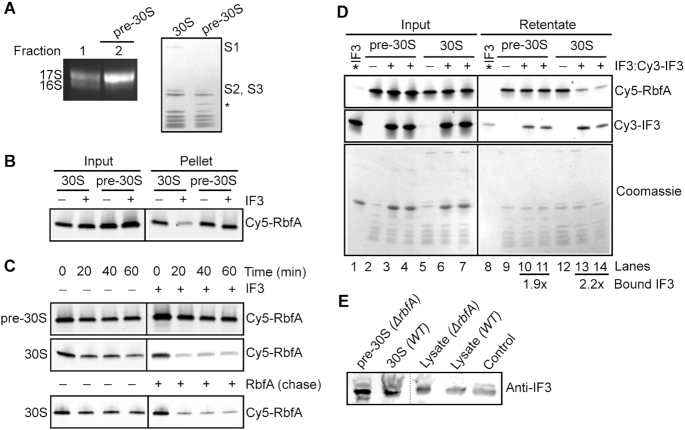
Figure 2.
IF3 cannot release RbfA from immature pre-30S subunits. (A) Composition of pre-30S ΔrbfA particles. Left, 1.5% agarose-TAE gel showing the rRNA profile. Fraction 2 containing >90% 17S pre-rRNA (pre-30S) was used for further assays. Right, 4–20% SDS PAGE comparing proteins from mature 30S and pre-30S ΔrbfA particles. Proteins S1, S2 and S3 are missing in the pre-30S particles. The identity of the extra band (*) is not known. (B) Pelleting assay; Cy5-RbfA (400 nM) was complexed with mature 30S or pre-30S subunits (200 nM) and challenged with IF3 (4 μM) as in Figure 1. (C) Kinetics of Cy5-RbfA dissociation from pre-30S and 30S subunits. Top, with or without 4 μM IF3; bottom, with or without 4 μM unlabeled RbfA. After excess Cy5-RbfA was removed from Cy5-RbfA•30S complexes by filtration (‘0 min’), complexes were filtered a second time to remove free Cy5-RbfA after an additional 20–60 min at 37°C. The 0 min samples that were filtered once contain ∼30% more Cy5-RbfA than the samples that were filtered twice. (D) Binding of Cy5-RbfA and Cy3-IF3 to pre-30S or 30S subunits, as in C. Top panel, Cy5-RbfA; middle panel, Cy3-IF3; lower panel, Coomassie stain. *IF3 (lanes 1 and 8); Cy3-IF3 only. Average fold change in bound IF3 over *IF3 background (lane 8) was 1.9 ± 0.15 in lanes 10 and 11 and 2.2 ± 0.9 in lanes 13 and 14; n = 2. Added IF3 was a mixture of 80% unlabeled IF3 and 20% Cy3-IF3. Cy5-RbfA was scanned with 600 PMT voltage, whereas Cy3-IF3 (Input) with 400 PMT voltage and Cy3-IF3 (retentate) with 500 PMT voltage. (E) Anti-IF3 western blot showing the presence of IF3 in the pre-30S and 30S fractions from BX41 (ΔrbfA) and BW25113 (WT) compared to ΔrbfA and WT lysates and purified IF3 (control).