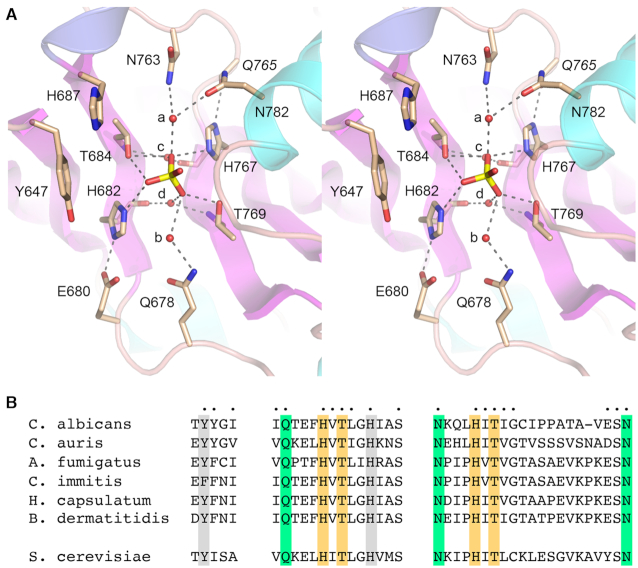
Figure 3.
CPD active site. (A) Stereo view of the CPD active site highlighting CPD interactions with the phosphate anion. Amino acids are shown as stick models with beige carbons. Waters are denoted by red spheres. Atomic contacts are indicated by dashed lines. (B) Conservation of three active site motifs among Trl1 CPD domains from six species of human pathogenic fungi: Candida albicans, Candida auris, Aspergillus fumigatus, Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis. Positions of amino acid side chain identity or similarity in all six proteins are indicated by dots above the C. albicans sequence. The phosphate-binding histidines and threonines are highlighted in gold shading. Amino acids making water-bridged contacts to the phosphate are in green shading. Two aromatic residues imputed to interact with the terminal nucleoside are shaded gray. The corresponding motifs in S. cerevisiae Trl1 CPD are shown at bottom.