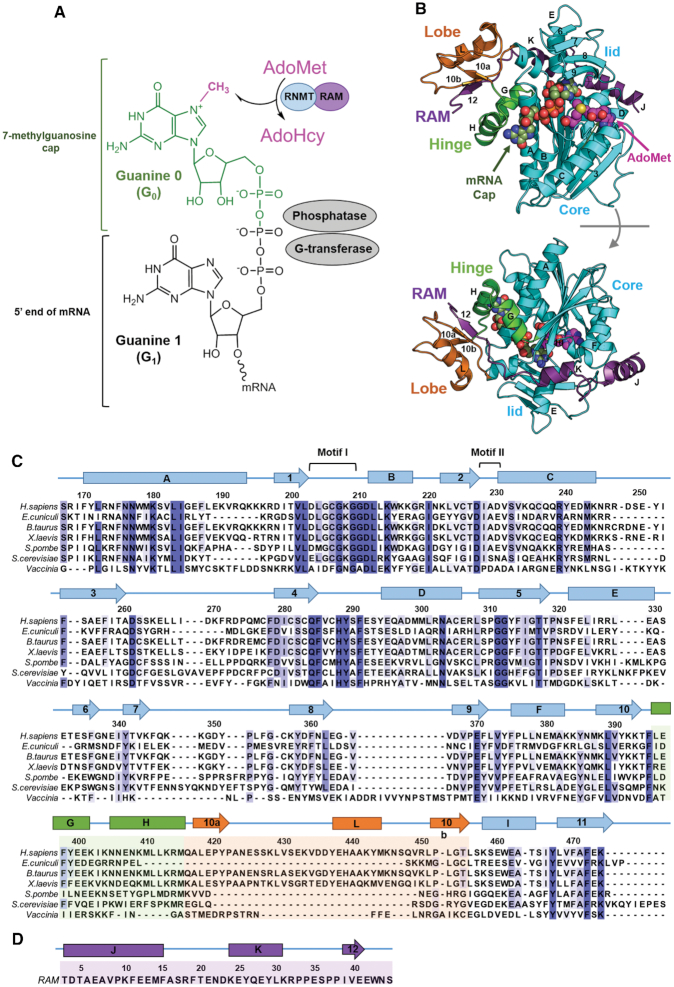
Figure 1.
mRNA cap and mRNA methyltransferases. (A) Structure of mRNA cap and mRNA capping enzymes. The G0, G1 and methyl (CH3) groups of the cap are labelled. Three enzymes involved in a stepwise formation of the cap, namely G-transferase, phosphatase and methyltransferase (RNMT), are indicated next to the respective chemical group. RNMT catalyses transfer of a methyl group from AdoMet to G0 at N7 position, producing a by-product AdoHcy. (B) Structure of the human RNMT–RAM complex (PDB ID: 5E8J). RNMT core (residues 165–476) is shown in cyan, the α-helix hinge (residues 395–415) in green and the modular lobe (residues 416–456) in orange. Nomenclature of key helices and strands is shown. RAM (residues 2–45) is displayed in violet. Representative binding poses of AdoMet and cap (G0pppG1) are shown using the spheres representation (also see Figure 3). (C) Multiple sequence alignment of human RNMT with its orthologs from a selection of eukaryotic organisms and viruses. The nomenclature of structural features shown was kept in line with Fabrega et al. (13) and Varshney et al. (8). The elements of the RNMT core are shown in cyan, α-helix hingein green, and modular lobe—in orange, consistent with (B). (D) Sequence and secondary structure elements of RAM (residues 2–45).