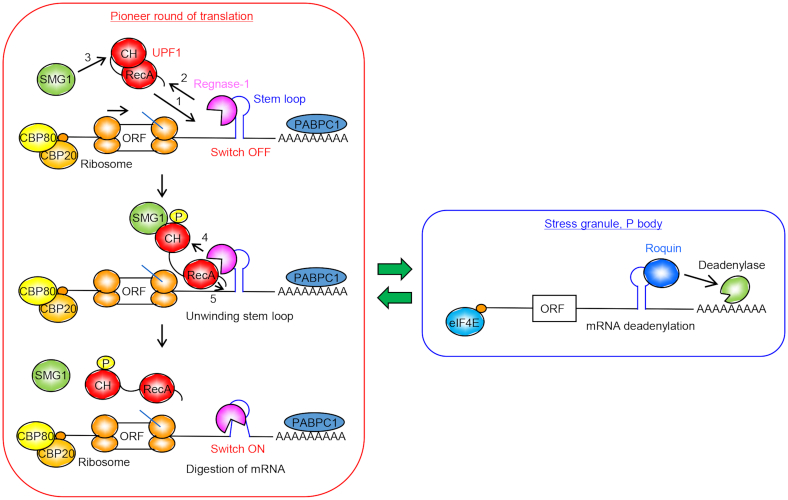
Figure 8.
A proposed model of mRNA degradation by Regnase-1 and Roquin. Regnase-1 recognizes stem–loop on 3′ UTR of inflammatory mRNAs prior to binding with UPF1 and translation. In the Regnase-1 pathway, SMG1 phosphorylates UPF1 at T28, which is supported by the association of Regnase-1-(90-130) linker region with UPF1 RecA helicase domain. The T28-phosphorylated N-terminal region of UPF1 is associated with Regnase-1 RNase domain (K257R258) to form stable interaction between Regnase-1 and UPF1. The association with Regnase-1 promotes UPF1 ATPase/helicase activity and the unwinding of stem–loop by UPF1 helicase activity induces RNA cleavage by Regnase-1. The SMG1-UPF1–Regnase-1 axis destabilizes inflammatory mRNAs bound by CBP80 during pioneer round of translation, whereas Roquin regulates mRNAs bound by eIF4E after steady-state round of translation. NCR, N-terminal conserved region; RecA, RecA-like domain.