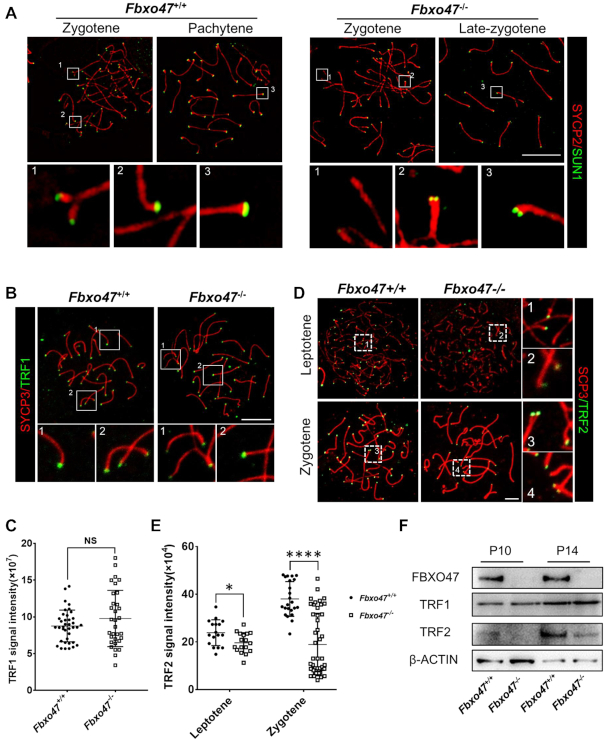
Figure 5.
Telomere-binding protein aberrations in FBXO47-deficient spermatocytes. (A) Spread spermatocytes from Fbxo47+/+ and Fbxo47−/− males were stained with anti-SYCP3 and anti-SUN1 antibodies. Scale bars, 10 μm. (B) Spread spermatocytes from Fbxo47+/+ and Fbxo47−/− males were stained with anti-SYCP3 and anti-TRF1 antibodies. Scale bars, 10 μm. (C) Graphs showing the TRF1 intensities per cells (n > 30 cells for each genotype). (D) Spread spermatocytes from Fbxo47+/+ and Fbxo47−/− males were stained with anti-SYCP3 and anti-TRF2 antibodies. Scale bars, 10 μm. (E) Graphs showing the TRF2 intensities per cells (n > 30 cells for each genotype). TRF1 signal intensity: Fbxo47+/+, 8.769 ± 0.351; Fbxo47−/−, 9.786 ± 0.6768; TRF2 signal intensity, leptotene: Fbxo47+/+, 23.873 ± 5.414; Fbxo47−/−, 19.660 ± 4.207; Zygotene: Fbxo47+/+, 38.046 ± 7.281; Fbxo47−/−, 18.953 ± 13.110. (F) FBXO47 is expressed in murine germ cells at P10 and P14. TRF2 is destabilized in Fbxo47-deficient germ cells. β-ACTIN served as a loading control.