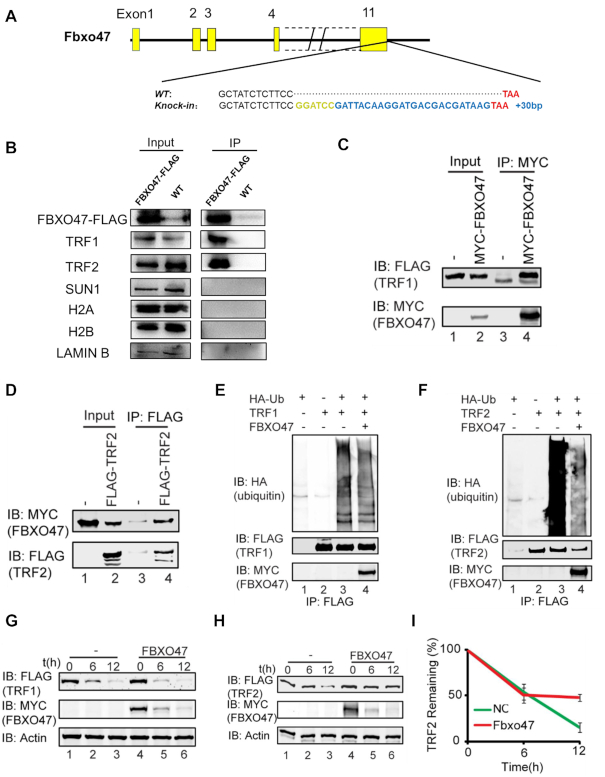
Figure 6.
Disruption of Fbxo47 destabilizes TRF2. (A) Schematic of FLAG-tagged alleles of endogenous Fbxo47 generated using CRISPR/Cas9. A 2-aa (Gly-Ser) spacer was added. (B) Co-IP analysis of FBXO47-FLAG and indicated proteins from Fbxo47-flag testicular protein extracts. (C and D) FBXO47 interacts with TRF1 and TRF2. pCS2-Myc-Fbxo47 and pRK-Flag-Trf1 or pRK-Flag-Trf2 were co-transfected into HEK293T cells. After 24 h, the cells were collected for immunoprecipitation (IP) with an anti-MYC (C) or anti-FLAG (D) antibody and analyzed with an anti-MYC and anti-FLAG antibodies. (E) The over-expression of FBXO47 has no influence on TRF1 ubiquitination. pCS2-Myc-Fbxo47, pRK-Flag-Trf1 and pCMV-HA-Ubiquitin were co-transfected into HEK293T cells. After 24 h, the cells were collected for immunoprecipitation (IP) with an anti-FLAG antibody and analyzed with anti-HA, anti-FLAG and anti-MYC antibodies. (F) FBXO47 impairs the ubiquitination of TRF2. pCS2-Myc-Fbxo47, pRK-Flag-Trf2 and pCMV-HA-Ubiquitin were co-transfected into HEK293T cells. After 24 h, cells were collected for immunoprecipitation (IP) with an anti-FLAG antibody and analyzed with anti-HA, anti-FLAG and anti-MYC antibodies. (G and H) FBXO47 stabilizes TRF2, rather than TRF1. Cycloheximide chase (CHX) assays of TRF1 (G) and TRF2 (H) were performed in FBXO47-over-expressing or empty vector-transfected samples. (I) Quantification of the relative TRF2 levels in (I).