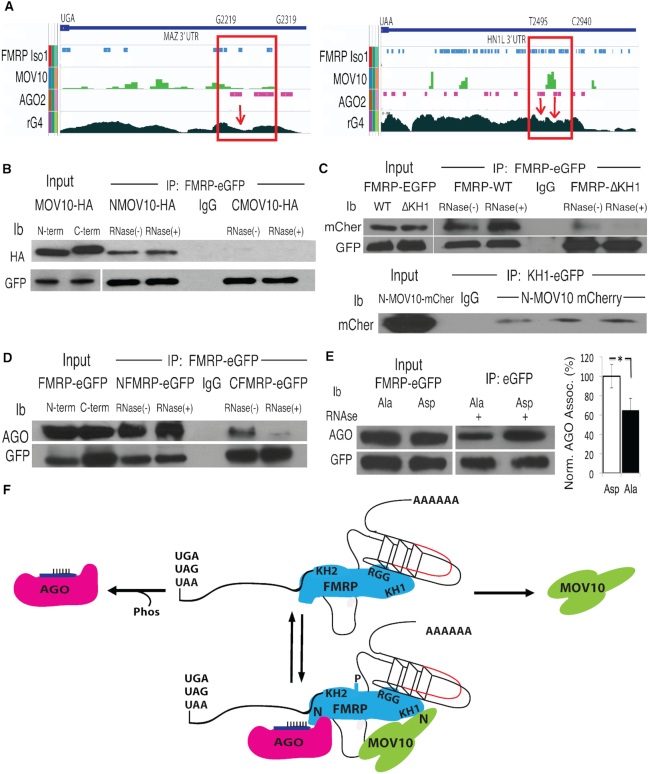
Figure 1.
FMRP binds the N-terminus of MOV10 through its KH1 domain and to AGO through its N-terminus in a phosphorylation dependent manner. (A) Integrated Genomics Viewer (IGV) screen shot of the 3′UTRs (stop codons indicated) of two protected mRNAs, MAZ and HN1L. CLIP regions in which FMRP, MOV10 and AGO2 binding are coincident with rG4s are shown in red boxes, indicated by nucleotide position at the top. rG4s, determined by rG4-seq, are troughs (indicated by red arrows). (B) A GFP-tagged FMRP construct was co-transfected with an HA-tagged N-terminal MOV10 (NMOV10-HA) or HA-tagged C-terminal MOV10 (CMOV10-HA) construct into HEK293 and immunoprecipitated (IP’ed) with an anti-eGFP antibody and examined by immunoblot (ib): antibody indicated on the left. IgG indicates the IP’ing antibody alone. RNase indicates treatment (+) or not (−) with RNase A. (C) (Top) eGFP-tagged KH1 domain of FMRP was IP’ed and examined for mCherry tagged N-terminal MOV10 (mCher). (Bottom) eGFP-tagged FMRP WT or ΔKH1 was IP’ed with anti-eGFP and examined for association with N-terminal MOV10 (mCher), three biological replicates are shown, (n = 3). (D) N-terminal half of FMRP (N-term through KH2 domain) or C-terminal half of FMRP (KH1 through C-terminus) was IP’ed and AGO association examined with the pan-AGO antibody 2A8, which recognizes the four AGO family members in the presence or absence of RNase A. (E) The primary phosphorylation site of murine FMRP (S499) was substituted to alanine (Ala) or aspartic acid (Asp) and association with AGO examined by eGFP-FMRP-IP. AGO co-IP was quantified by normalizing to AGO and transgene protein levels, (n = 3), error bars represent SD, *P < 0.05, Student t-test. (F) Based on their respective interacting domains, a representation of a dynamic RNP complex involving FMRP, MOV10 and AGO proteins in the 3′ UTR of an mRNA (stop codons indicated) bearing an RNA G-Quadruplex (rG4) with an MRE site (red). N denotes N-terminus. All experiments were performed in triplicate, representative immunoblots are shown.