Abstract
Most studies investigating the effect of childhood trauma on the brain are retrospective and mainly focus on maltreatment, whereas different types of trauma exposure such as growing up in a violent neighborhood, as well as developmental stage, could have differential effects on brain structure and function. The current magnetic resonance imaging (MRI) study assessed the effect of trauma exposure broadly and violence exposure more specifically, as well as developmental stage on the fear neurocircuitry in 8–14-year-old children and adolescents (N=69). We observed reduced hippocampal and increased amygdala volume with increasing levels of trauma exposure. Second, higher levels of violence exposure were associated with increased activation in the amygdala, hippocampus and vmPFC during emotional response inhibition. This association was specifically observed in children younger than 10 years. Finally, increased functional connectivity between the amygdala and brainstem was associated with higher levels of violence exposure. Based on the current findings, it could be hypothesized that trauma exposure during childhood results in structural changes that are associated with later risk for psychiatric disorders. At the same time, it could be postulated that growing up in an unsafe environment leads the brain to functionally adapt to this situation in a way that promotes survival, where the long-term costs or consequences of these adaptations are largely unknown and an area for future investigations.
Keywords: childhood trauma, response inhibition, amygdala, hippocampus, ventromedial prefrontal cortex (vmPFC), functional magnetic resonance imaging (fMRI), brain structure
Introduction
Childhood adversity has been shown to increase risk for psychiatric disorders across the lifespan, including posttraumatic stress disorder (PTSD; Dunn, 2016; McLaughlin, 2010; McLaughlin, 2012; Norman, 2012; Widom, 2007) and depression (Dunn, 2016). One of the primary mechanisms by which childhood trauma is theorized to contribute to PTSD and similar pathologies is through alterations to the fear neural circuitry (Jovanovic & Ressler, 2010); however, the influence of trauma exposure on this circuitry during development is not fully understood.
The primary brain regions involved in the fear inhibition circuit are the amygdala, hippocampus, and ventromedial prefrontal cortex (vmPFC). The amygdala serves as an essential locus for fear acquisition, memory, and expression (Kim & Jung, 2006). Increased amygdala activation has been demonstrated in adults reporting childhood maltreatment (Dannlowski, 2013; Dannlowski, 2012; Grant, 2011; van Harmelen, 2013) as well as in maltreated or neglected children (Maheu, 2010; McCrory, 2013; Suzuki, 2014; Tottenham, 2011). Most structural MRI studies in children or adolescents with Adverse Childhood Events (ACEs) reported decreased amygdala volumes (Edmiston, 2011; Hanson, 2015; Luby, 2019; McLaughlin, 2016), though larger volumes were observed in previously institutionalized children (Mehta, 2009; Tottenham, 2010). The hippocampus functions to contextualize memory and learning, and is thought to contribute to inhibition based on contextual information (Milad, 2007). Increased hippocampal activation to threatening faces has been observed in maltreated children (Maheu, 2010). Reduced hippocampal volume has repeatedly been demonstrated in adults reporting childhood trauma (e.g. (Bremner, 2003; Stein, 1997; Teicher, 2012; Vythilingam, 2002) and in a small number of studies that investigated children or adolescents with ACEs (Hanson, 2015; Luby, 2019; McLaughlin, 2016; Paquola, 2017; Rao, 2010). Via a close interconnection with the amygdala, the vmPFC modulates and inhibits amygdala-expressed fear responses (Milad & Quirk, 2012; Phelps, 2004; Stevens, 2013). Where retrospective studies showed decreased vmPFC activation in adults with childhood adversity (Stevens, 2016; van Harmelen, 2014), studies in adolescents with early-life stress showed increased vmPFC activation on response inhibition tasks (Carrion, 2008; Mueller, 2010). Decreased resting state amygdala-vmPFC and hippocampus-vmPFC connectivity was observed in maltreated adolescents compared to controls (Herringa, 2013). Taken together, there is substantial evidence that childhood trauma impacts the fear neurocircuitry.
Inhibition also takes place on a cognitive level via response inhibition, where a learned response must be suppressed. A Go/NoGo task is frequently used to measure response inhibition, and trauma exposure and PTSD have been associated with impaired brain responses on this task (Falconer, 2008; Jovanovic, 2013; Stevens, 2016; van Rooij, 2016; van Rooij, 2018). Interestingly, impairments were found in fear inhibition regions such as the vmPFC (Jovanovic, 2013; Stevens, 2016) and hippocampus (van Rooij, 2016; van Rooij, 2018), and several prior studies used (emotional) response inhibition tasks to show alterations in the fear neurocircuitry in maltreated children (Carrion, 2008; Mueller, 2010; Tottenham, 2011).
The majority of prior studies have focused on childhood maltreatment or neglect, whereas much less is known about the effects of exposure to other types of trauma, such as violence exposure. The Grady Trauma Project is an ongoing study of PTSD risk factors in a low-income population in Atlanta, GA. Most participants live in unsafe neighborhoods in inner-city Atlanta and report high levels of trauma exposure, and PTSD and depression symptoms (Gillespie, 2009). While trauma research has focused extensively on the effects of continuous exposure to stress and trauma in an unsafe environment as part of military deployment, little is known about the neurobiological consequences of growing up in a dangerous environment and being exposed to violence on a regular basis as a normal part of life. The current study focused on the children of our adult participants to assess the effect of trauma and violence exposure during development on the fear neurocircuitry.
In line with retrospective structural MRI studies, we hypothesized that more trauma exposure would be negatively associated with hippocampal and amygdala volume. Following MRI studies suggesting associations specifically between left hippocampal volumes and PTSD symptoms (Nelson 2017) or major or bipolar depression (MacMaster 2014), we analyzed structural findings per hemisphere. Second, we expected to see a positive correlation between both trauma exposure broadly, and violence exposure more specifically, with amygdala, hippocampal and vmPFC activation during a Go/NoGo task that measured response inhibition in an emotional context. Analyses were performed for bilateral structures as there were no specific hypotheses regarding functional laterality. Finally, functional connectivity analyses were conducted to investigate if trauma or violence exposure was associated with increases in functional connectivity within the fear neurocircuitry, presented in the Supplementary Materials.
In addition to a paucity of investigation of different types of trauma, few studies have assessed the effect of developmental stage. Using retrospective recall, age 9 was identified as the age when the impact of the trauma on the fear neurocircuitry was largest, for example Teicher et al demonstrated differential effects of age and type of trauma on hippocampal and amygdala volumes (Teicher 2016; 2018). Furthermore, Gee and colleagues (2013) have found evidence of a developmental shift in neural (stimulus-elicited) connectivity around age 10, such that positive functional connectivity between the amygdala and PFC shifts to a negative connectivity pattern (Gee, 2013). Studies on sensitive periods in brain development and physiology revealed this age as a critical window in which environmental influences, such as trauma exposure, can induce long-lasting neurobiological effects (Glenn, 2012; Jovanovic, 2014). In addition to including age as a continuous covariate in the correlation analyses, we stratified by age group to assess the differential patterns of violence exposure within each age category. We therefore performed secondary analyses to assess the effect of this critical period by splitting the children in younger than 10 and 10 and older, and hypothesized that trauma exposure differentially effects the fear neurocircuitry in the two age groups.
Materials and Methods
Participants
African American children and adolescents age 8–14 years (N=69, 36 female) were recruited through the Grady Trauma Project (Gillespie, 2009). Exclusion criteria for children were a history of bipolar disorder or schizophrenia, active psychotic symptoms, or cognitive disability, previous head injury with loss of consciousness, history of stroke, epilepsy, neurological disorder, autism spectrum disorder, or brain tumor, metal in the body, or hearing or vision impairment unable to be corrected by glasses.
Testing took place at Grady Memorial Hospital and the scan at Facility for Education and Research in Neuroscience at Emory University. The protocol was approved by the Institutional Review Boards of Emory University and Research Oversight Committee at Grady Memorial Hospital. Written consent was obtained from a legal guardian of the child participant and oral (younger than 11) or written (ages 11 and older) consent was obtained from child participants.
Trauma and Violence Assessment
Interviews were conducted with each child to assess for trauma exposure broadly, violence exposure more specifically, as well as PTSD, anxiety and depression symptoms. Child-reported trauma exposure was assessed with the Traumatic Events Screening Inventory (TESI) for children (Ribbe, 1996). This 19-item questionnaire assessed a variety of potential traumatic events, such as disasters, accidents, injuries, violence and abuse, and was answered with Yes or No to each item. The total score was used in the analyses as a measure for trauma exposure broadly. Exposure to violence specifically was measured using the Violence Exposure Scale for Children-Revised (VEX-R)(Fox & Leavitt, 1995), which specifically assesses exposure to violent events. The 25-item questionnaire has a male and female version of drawings that accompany questions and a frequency rating scale to inquire about exposure to violence in the home, school, and community. This assessment has been previously used with children from the Grady Trauma Project demonstrating high rates of violence exposure (Cross, 2018). Current PTSD symptoms were assessed using the child-report UCLA PTSD Reaction Index (UCLA-RI) (Steinberg, 2004). The Behavioral Assessment System for Children (BASC) was used to assess anxiety and depression symptoms (Reynolds 2011). The BASC score is a gender- and age-corrected t-value with 50 indicating the mean.
Emotional Go/NoGo fMRI Task
The emotional Go/NoGo (eGNG) task has been previously used by Tottenham (2011) in a study with children with early life stress. Participants were instructed to press a button for fearful faces (Go trial) and withhold responses for neutral faces (NoGo trial), or vice versa, and the order of runs was counterbalanced among participants. Each stimulus was followed by a varied inter-trial interval ranging from 2500 to 15000ms. There were two runs with 36 Go trials and 12 NoGo trials in each run.
Overall reaction time, % correct Go’s, % correct NoGo’s and accuracy [correct Go’s–incorrect NoGo’s)/total number of trials] were calculated for behavioral analyses. The contrast for correct NoGo trials larger than Go trials was used for the fMRI analyses. Only accurate trials were included in the imaging analyses to ensure proper engagement of participants with the task.
MRI Procedures and Analyses
Participants completed a mock scan protocol at a visit prior to their actual scan during which they were acclimated to the scanner. Participants completed practice sessions of the study tasks both inside and outside of the mock scanner to ensure that they understood how to complete the task.
Functional and structural MRI scans were acquired on a 3.0-T Siemens Trio (whole-body) MR scanner using a 32-channel head coil. A T1-weighted image (176 slices, TR= 2250ms TE= 4.18ms and voxel size 1×1×1mm) was used for within-subject registration and to measure left and right hippocampal and amygdala volumes. Structural T1-weighted MRI scans were analyzed using Freesurfer v6.0. Quality control and processing were performed in conjunction with standardized ENIGMA protocols (http://enigma.ini.usc.edu). Left and right hippocampal and amygdala volumes, and intracranial volumes were extracted and exported to SPSS 26.0.
Two runs of 131 echo planar imaging (EPI) blood oxygen level dependent (BOLD) images (total of 262) were acquired during which the participants performed the emotional Go/NoGo task. Volumes contained 44 slices of 2.5mm thickness acquired in a descending sequential slice order parallel to the anterior-posterior commissure line, with a 0.5mm slice gap. GRAPPA parallel imaging with an acceleration factor of 2 was used to facilitate speed of acquisition. The following parameters were used: repetition time (TR)=2330ms, echo time (TE)=30ms, flip angle= 90 degrees, and voxel size 3×3×3mm.
Functional images were analyzed (file conversion, image preprocessing and statistical analyses) using Statistical Parametric Mapping, version 8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm/). ArtRepair was used to detect and repair bad slices. Functional images were slice-time corrected and realigned to the first image in the session to correct for motion. Next, ArtRepair was used to detect and repair bad volumes and to test if participants exceeded the motion threshold set for exclusion (>2mm/TR). The average maximum (framewise) motion was 1.06mm/TR. Bad volumes were interpolated, a maximum of 10% per participant with an average of 3.9% for included participants. The structural T1 volume was co-registered to the mean of the realigned functional images and spatially normalized to standardized Montreal Neurological Institute (MNI) space. The normalization parameters were then applied to the functional volumes and the images smoothed with a 6mm full-width at half maximum Gaussian kernel.
Subject-level statistical maps were created for the correct NoGo>Go contrast. Data were extracted for a priori regions of interest (ROIs) and as there were no specific hypotheses for unilateral analyses, bilateral ROIs were extracted in accordance with prior studies (Stevens, 2014; van Rooij, 2018): bilateral amygdala based on the Anatomical Automatic Labeling (AAL) atlas (http://www.gin.cnrs.fr/AAL); bilateral hippocampus based on the Hammers atlas (Rodionov, 2009) and the vmPFC, for which a 6mm sphere around a peak voxel (MNI coordinates: 4, 44, −4) from a previous response inhibition study showing vmPFC activation (Jovanovic, 2013). ROI data were exported to SPSS.
Group Analyses
For both trauma exposure broadly (TESI) and violence exposure specifically (VEX-R frequency) we performed correlation analyses with behavioral data, structural measures of the hippocampus and amygdala, and functional measures of bilateral hippocampal, amygdala and vmPFC activation. Second, partial correlations correcting for age, sex, and intracranial volume (ICV) for structural measures were performed.
Exploratory regression analyses were performed for significant correlations to assess the effect of age group (<10 and >=10) by creating interaction terms for trauma or violence exposure * age group and adding them to the model along with main effects.
Whole brain analyses and functional connectivity analyses were performed, and methods, results and discussion of the findings are presented in the Supplementary Materials. Additional correlation analyses with PTSD symptoms and regression analyses assessing the differential effect of sex are also presented in the Supplementary Materials.
Results
Participants
Sixty-nine African American children and adolescents (8 to 14 years) were scanned (Table 1) and data from these participants was included in the structural analyses, functional analyses or both, resulting in different analytical datasets. Structural data of 6 participants was unusable for analyses due to motion, resulting in N=63 for the structural analyses. Functional eGNG data was collected on 66 participants, however, 15 participants exceeded the motion threshold of 2mm/TR, 2 participants fell asleep during the scan, and 2 participants did not (correctly) press any buttons, resulting in a sample of N=47. Behavioral response inhibition data was available for 62 participants. No significant differences were observed in demographics for participants included in the structural and functional analyses. Age (in months) and sex were included as covariates in secondary analyses.
Table 1.
Demographics and clinical data
| N=69 | Mean | SD | Range | Percentage |
|---|---|---|---|---|
| Age (in months) | 130.0 | 19.4 | 99–177 | |
| Sex (% females) | 52.2% | |||
| Household income (% < 2019 federal poverty level) | 76.9% | |||
| Global Trauma exposure (TESI) | 5.5 | 3.4 | 0–18 | |
| Violence exposure (VEX-R) | 14.1 | 8.3 | 1–44 | |
| PTSD symptoms | 14.7 | 11.5 | 0–41 | |
| Meet for PTSD (DSM) | 15.9% | |||
| Anxiety symptoms T-score (BASC) | 48.4 | 12.0 | 29–77 | |
| >1SD above mean | 14.5% | |||
| Depression symptoms T-score (BASC) | 50.5 | 10.15 | 37–80 | |
| >1SD above mean | 16.1% |
Behavioral findings
The group means for accuracy, % correct Go’s, % correct NoGo’s, and overall Go reaction time are presented in Table 2. The means for the N=62 participants did not significantly differ from means of the N=47 included in the functional MRI analyses.
Table 2.
Behavioral data
| N=62 (behavioral data available) | N=47 (included in fMRI analyses) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Accuracy (%) | 77.3 | 15.8 | 77.0 | 17.4 |
| Correct Go’s (%) | 85.4 | 16.2 | 84.0 | 17.5 |
| Correct NoGo’s (%) | 75.7 | 19.2 | 78.8 | 15.4 |
| Go reaction time (ms) | 830.4 | 204.6 | 832.9 | 212.5 |
There were no significant correlations between trauma exposure and the behavioral measures. There was a significant negative correlation of violence exposure with reaction time (r=−0.34, p=0.008), however, after correcting for age and sex, this effect was no longer significant (r=−0.14, p=0.263). Follow-up analyses showed a positive correlation between age and violence exposure (r=0.32, p=0.008), and a negative correlation between age and reaction time (r=−0.51, p<0.001).
Trauma exposure
More trauma exposure correlated with smaller left and right hippocampal volume (Table 3a, Figure 1). After correcting for ICV, age and sex, the negative correlation with the left hippocampus remained significant, and a positive correlation with the left amygdala was observed. Only the correlations with the left hippocampus survived correction for multiple comparisons. There was no effect of trauma exposure on the functional measures.
Table 3.
Correlation analyses for trauma and violence exposure with structural and functional MRI measures
| Brain volumes | Inhibition-related activation | |||||||
|---|---|---|---|---|---|---|---|---|
| L HPC | R HPC | L AMYG | R AMYG | bil HPC | bil AMYG | vmPFC | ||
| a. Trauma exposure | r | −0.33 | −0.26 | 0.07 | −0.05 | 0.12 | 0.13 | 0.07 |
| p | 0.008 | 0.042 | 0.607 | 0.672 | 0.408 | 0.400 | 0.621 | |
| corrected for age, sex (and ICV for volumes) | r | −0.35 | −0.20 | 0.26 | 0.14 | 0.12 | 0.14 | 0.12 |
| p | 0.006 | 0.125 | 0.046 | 0.296 | 0.451 | 0.350 | 0.441 | |
| b. Violence exposure | r | 0.04 | −0.01 | 0.20 | 0.15 | 0.39 | 0.30 | 0.27 |
| p | 0.732 | 0.965 | 0.118 | 0.23 | 0.007 | 0.039 | 0.064 | |
| corrected for age, sex (and ICV for volumes) | r | −0.01 | −0.05 | 0.24 | 0.21 | 0.39 | 0.34 | 0.35 |
| p | 0.934 | 0.731 | 0.064 | 0.113 | 0.009 | 0.024 | 0.017 | |
ICV, intracranial volume
Figure 1. Correlation analyses of trauma exposure with brain structure.
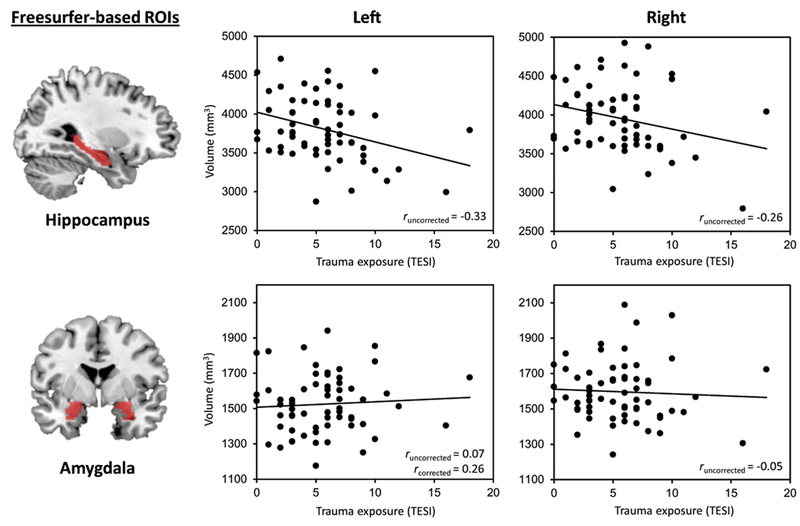
Figure 1 displays the correlation between trauma exposure measured as number of traumas with the TESI, and hippocampal and amygdala volume (N=63). The figures display the uncorrected correlation analyses, the results from the corrected analyses (correcting for intracranial volume (ICV), age and sex) can be found in Table 3. Note that the association between trauma exposure and the left amygdala was only significant after correcting for ICV, age and sex.
Violence exposure
More violence exposure correlated with more activation in the bilateral hippocampus and amygdala, and marginally with the vmPFC (Table 3b, Figure 2). After correcting for age and sex, all 3 correlations were significant. Only the correlation with bilateral hippocampal activation survived correction for multiple comparisons. No correlations were observed between violence exposure and structural measures.
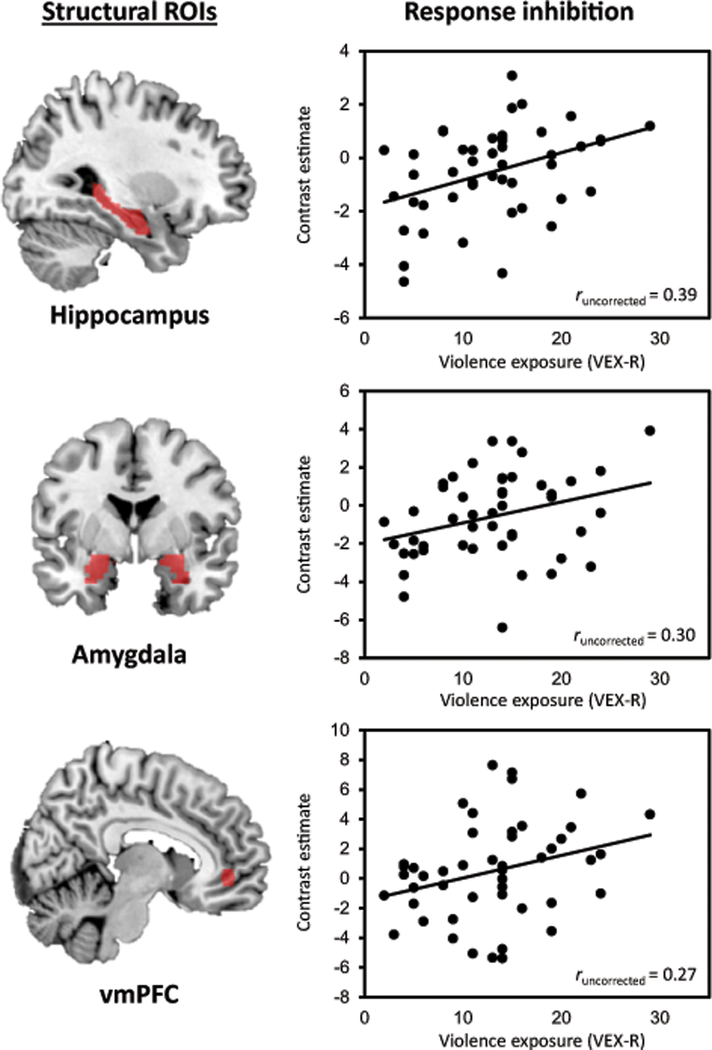
Figure 2. Correlation analyses of violence exposure with brain function.
Figure 2 displays the correlation between violence exposure measured as frequency of exposure to violence with the VEX-R, and hippocampal, amygdala, and vmPFC activation as measured during response inhibition (NoGo>Go trials). The figures display the uncorrected correlation analyses, the corrected analyses (correcting for age and sex) can be found in Table 3.
Exploratory regression analyses were performed to assess the effect of age group (<10, N=15 vs. >=10, N=32) on the relation between violence exposure and functional outcomes, because significant correlations were observed in the initial analyses. A significant interaction between age group and violence exposure was observed for hippocampal activation (F(3,46)=4.63, p=0.007; interaction, t=−2.08, p=0.043) and amygdala activation (F(3,46)=3.59, p=0.021; interaction, t=−2.42, p=0.020), such that significant correlations were only observed in younger children (hippocampus, r=0.59, p=0.037; amygdala, r=0.75, p=0.003).
Discussion
The current study in 69 children and adolescents showed that more trauma exposure in general (including multiple types of trauma) was associated with structural changes in the hippocampus and amygdala, whereas more violence exposure specifically correlated with more functional changes in the amygdala, hippocampus and vmPFC, particularly in children younger than 10 years of age. Furthermore, more violence exposure was related to stronger functional connectivity between the left amygdala and the brainstem (in Supplementary Materials).
As hypothesized, more trauma exposure was associated with smaller (left) hippocampal volume. This effect has been demonstrated repeatedly in prior adult retrospective studies and a few pediatric studies, but here we build on prior work by showing this effect can already be observed during development in a non-clinical sample of children and adolescents. This is important given that reduced hippocampal volume, in turn, has been shown to be a risk factor for the development of PTSD (Gilbertson, 2002) and depression (Rao, 2010). We also showed that only trauma exposure more broadly, but not violence exposure specifically, was related to reduced hippocampal volume. Amygdala volume was found to be positively correlated with trauma exposure, which parallels studies in previously institutionalized children (Mehta, 2009; Tottenham, 2010), but contradicts studies on maltreatment showing reduced amygdala volume (Edmiston, 2011; Hanson, 2015; Luby, 2019; McLaughlin, 2016). However, as our amygdala finding did not survive correction for multiple comparisons, replication is warranted before further interpretation.
Further, increased levels of violence exposure were associated with more activation in the amygdala, hippocampus and vmPFC during emotional response inhibition. These findings parallel studies in maltreated or neglected children who demonstrated more amygdala activation compared to controls (Maheu, 2010; McCrory, 2013; Tottenham, 2011), and increased hippocampal activation (Maheu, 2010) to emotional faces. Our findings also correspond with increased mPFC activation observed on a Go/NoGo task in children with trauma exposure and PTSS (Carrion, 2008) and adolescents with early life stress (Mueller, 2010). However, these previous studies only demonstrated group differences and did not show a continuous association between trauma exposure and brain function as we have demonstrated. Only Suzuki et al (2015) showed a dose-response relation between number of cumulative stressful and/or traumatic life events and amygdala, sgACC and hippocampal activation in response to emotional faces. Furthermore, in a retrospective study in adults, more childhood trauma was found to positively correlate with hippocampal activation during a Go/NoGo task, but only in individuals with the COMT Val/Val genotype (van Rooij, 2016), suggesting the need for further assessment of genetic influences.
A possible explanation for the positive correlation between violence exposure and amygdala, hippocampus and vmPFC activation is that children with high levels of violence exposure show an appropriate, increased attention-directing response. Following prior work by Tottenham (2011), it could be suggested that increased amygdala activation is a manifestation of increased vigilance to emotional stimuli, provoked by exposure to violence in our population. Based on prior studies on the role of the hippocampus and vmPFC in fear regulation, it can be hypothesized that increased hippocampal recruitment could help children contextualize experiences, and augmented prefrontal control could regulate fear accordingly. Therefore, increased fear neurocircuitry activation with higher levels of exposure to violence may reflect an adaptive brain response to growing up in a dangerous, violent environment, especially since this association in our non-clinical population is only observed for violence exposure specifically, and not trauma exposure in general.
Levels of psychopathology in our population were relatively low considering the high levels of trauma exposure. Moreover, there was no relation between PTSD symptoms and brain structure or function (in Supplementary Materials). Importantly, while heightened neural activation may be an adaptive mechanism during childhood in an adverse environment, the long-term potentially excitotoxic effects of this over-engagement of the fear neurocircuitry and its risk for later psychopathology are not clear. It is possible that chronic, adaptive vmPFC and hippocampal overactivation in childhood lead to maladaptive vmPFC and hippocampal underactivation in adulthood, patterns observed in adults with PTSD (Jovanovic, 2013; van Rooij, 2016). This pattern was suggested by Tarullo and Gunnar (2006) as the explanation for why maltreated children over-secrete cortisol while adults with childhood maltreatment under-secrete cortisol. Yet, as our study population is not a clinical population, many of the participants may become resilient adults and these brain alterations may promote this as suggested in (van Rooij, 2016). Therefore, based on this study we cannot conclude whether the observed correlation between violence exposure and brain activation is a marker of future resilience or psychiatric risk.
As hypothesized, we observed an effect of developmental stage on our functional outcomes. Variability in the amygdala, hippocampus and vmPFC activation was tightly linked with violence exposure specifically in younger children. This could be explained by a variety of maturation processes, such as critical periods for brain development (Knudsen, 2004), prefrontal maturation during adolescence (Caballero, 2016), changes in amygdala-vmPFC functional and structural connectivity (Gee, 2013; Jalbrzikowski, 2017), improvements in safety signal processing in older children (Jovanovic, 2014), and changes in social functioning, though these hypotheses require further exploration. Notably, the sample size of the younger children was relatively small (N=15) and replication in a larger sample is warranted. However, this is a very difficult sample to collect and therefore largely understudied, and much needed data to be added to the literature.
Other limitations of this study included that it is a cross-sectional study, and therefore no directional conclusions can be drawn from this data, and it is unclear if the associations we observed between brain function and violence exposure indicate a long-term protective or harmful effect. Second, the participants have a somewhat wide age range (8–14) across pre- and post-pubertal developmental stages. Though we assessed the effects of age by stratifying participants by age group (<10 and >=10) and included age in months as a covariate in our secondary analyses, it would be informative to more carefully control for age and pubertal stage in future studies. Third, the findings in our high-risk African-American population from inner-city Atlanta may not generalize to other populations. Finally, many children who grow up in an unsafe environment live below the poverty line. Research has shown pervasive effects of poverty on brain structure (Hair, 2015), including the hippocampus and amygdala (Luby, 2013). In this study we did not separately assess the effects of poverty, because the majority of our participants were from low income families with little variation to include in the analyses. On the other hand, this population therefore better allowed us to examine the effects of trauma and violence exposure than in studies with a wide income range where poverty effects can confound the effects of trauma/violence.
Conclusion
In this neuroimaging study in an at-risk pediatric population ages 8–14, we observed (1) an association between structural brain alterations and childhood trauma more generally, and (2) functional changes which correlated with violence exposure specifically. Based on the current findings, it could be hypothesized that general trauma exposure during childhood results in structural changes in the hippocampus (and amygdala) that are associated with later risk for psychiatric disorders. At the same time, it can be postulated that growing up in an unsafe environment with high levels of violence exposure leads the brain to functionally adapt to this situation in a way that promotes survival, where the long-term costs or consequences of these adaptations are largely unknown and an area for future investigations. Given the importance of the fear neurocircuitry for psychiatric disorders, increased understanding of the effects of trauma exposure on the developing brain is essential for early detection of individuals at risk for developing psychiatric disorders.
Supplementary Material
Acknowledgements
We like to thank Bekh Bradley, Rebecca Hinrichs, Angelo Brown, Alexander Vance, Ye Ji Kim, Vasiliki Michopoulos, Abigail Powers, and the staff and volunteers of the Grady Trauma Project.
Funding This project was funded by the National Institute of Mental Health (MH111682 and MH100122 to TJ, and 2R01MH091864 to NT) and the Brain and Behavior Research Foundation (NARSAD; to TJ).
Footnotes
Conflict of interest Authors report no conflict of interest.
Data sharing Part of the data is shared through RDoCdb (NIH data archive). The other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, … Charney DS (2003). MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry, 160(5), 924–932. doi: 10.1176/appi.ajp.160.5.924 [DOI] [PubMed] [Google Scholar]
- Caballero A, Granberg R, & Tseng KY (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience and biobehavioral reviews, 70, 4–12. doi: 10.1016/j.neubiorev.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, & Reiss AL (2008). Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety, 25(6), 514–526. doi: 10.1002/da.20346 [DOI] [PubMed] [Google Scholar]
- Cross D, Vance LA, Kim YJ, Ruchard AL, Fox N, Jovanovic T, & Bradley B. (2018). Trauma exposure, PTSD, and parenting in a community sample of low-income, predominantly African American mothers and children. Psychological trauma : theory, research, practice and policy, 10(3), 327–335. doi: 10.1037/tra0000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, … Suslow T. (2013). Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp, 34(11), 2899–2909. doi: 10.1002/hbm.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H. (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry, 71(4), 286–293. doi: 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Dunn EC, Nishimi K, Powers A, & Bradley B. (2016). Is developmental timing of trauma exposure associated with depressive and post-traumatic stress disorder symptoms in adulthood? J Psychiatr Res, 84, 119–127. doi: 10.1016/j.jpsychires.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, & Blumberg HP (2011). Corticostriatal-Limbic Gray Matter Morphology in Adolescents With Self-reported Exposure to Childhood Maltreatment. JAMA Pediatrics, 165(12), 1069–1077. doi: 10.1001/archpediatrics.2011.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, … Williams LM (2008). The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry and Neuroscience, 33(5), 413–423. [PMC free article] [PubMed] [Google Scholar]
- Fox N, & Leavitt L. (1995). The Violence Exposure Scale for children-VEX (preschool version). College Park: Department of Human Development, University of Maryland. [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N. (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(10), 4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. (2012). The development of fear learning and generalization in 8–13 year-olds. Developmental psychobiology, 54(7), 675–684. doi: 10.1002/dev.20616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, & Pitman RK (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247. doi: 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, … Ressler KJ (2009). Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry, 31(6), 505–514. doi: 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, & Shelton R. (2011). Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res, 45(7), 886–895. doi: 10.1016/j.jpsychires.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatrics, 169(9), 822–829. doi: 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ (2015). Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological Psychiatry, 77(4), 314–323. doi: 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A, 110(47), 19119–19124. doi: 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, & Luna B. (2017). Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biological Psychiatry, 82(7), 511–521. doi: 10.1016/j.biopsych.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, … Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. doi: 10.1016/j.cortex.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, & Bradley B. (2014). Development of fear acquisition and extinction in children: effects of age and anxiety. Neurobiology of learning and memory, 113, 135–142. doi: 10.1016/j.nlm.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, & Ressler KJ (2010). How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. American Journal of Psychiatry, 167(6), 648–662. doi: 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Jung MW (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and biobehavioral reviews, 30(2), 188–202. doi: 10.1016/j.neubiorev.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI (2004). Sensitive Periods in the Development of the Brain and Behavior. Journal of Cognitive Neuroscience, 16(8), 1412–1425. doi: 10.1162/0898929042304796 [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D. (2013). The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatrics, 167(12), 1135–1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Tillman R, & Barch DM (2019). Association of Timing of Adverse Childhood Experiences and Caregiver Support With Regionally Specific Brain Development in Adolescents. JAMA Network Open, 2(9), e1911426-e1911426. doi: 10.1001/jamanetworkopen.2019.11426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, … Ernst M. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, affective & behavioral neuroscience, 10(1), 34–49. doi: 10.3758/CABN.10.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, … Viding E. (2013). Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry, 202(4), 269–276. doi: 10.1192/bjp.bp.112.116624 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green J, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: Associations with persistence of DSM-IV disorders. Archives of General Psychiatry, 67(2), 124–132. doi: 10.1001/archgenpsychiatry.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of us adolescents. Archives of General Psychiatry, 69(11), 1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, … Pine DS (2016). Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology, 41(8), 1956–1964. doi: 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster FP, Carrey N, Langevin LM, Jaworska N, Crawford S. (2014). Disorder-specific volumetric brain difference in adolescent major depressive and bipolar depression. Brain Imaging Behavior, 8(1), 119–1127. Doi: 10.1007/s11682-013-9264-x. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, … Sonuga-Barke EJ (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry, 50(8), 943–951. [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol, 63, 129–151. doi: 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry, 62(5), 446–454. doi: 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, … Ernst M. (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia, 48(10), 3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, and Tumpap AM (2017). Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: a meta-analytic study. CNS Spectrums, 22(4), 363–372. Doi: 10.1017/S1092852916000833 [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, & Vos T. (2012). The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med, 9(11), e1001349. doi: 10.1371/journal.pmed.1001349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C, Bennett MR, Hatton SN, Hermens DF, Groote I, & Lagopoulos J. (2017). Hippocampal development in youth with a history of childhood maltreatment. Journal of Psychiatric Research, 91, 149–155. doi: 10.1016/j.jpsychires.2017.03.019 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43(6), 897–905. doi: 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, & Hammen CL (2010). Hippocampal Changes Associated with Early-Life Adversity and Vulnerability to Depression. Biological Psychiatry, 67(4), 357–364. doi: 10.1016/j.biopsych.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, & Vannest KJ (2011). Behavioral assessment system for children (BASC) In Encyclopedia of clinical neuropsychology (pp. 366–371): Springer. [Google Scholar]
- Ribbe D. (1996). Psychometric review of Traumatic Event Screening Instrument for Children (TESI-C) In B. H. S (Ed.) (Ed.), Measurement of stress, trauma, and adaptation (pp. 386–387). Lutherville: MD: Sidran Press. [Google Scholar]
- Rodionov R, Chupin M, Williams E, Hammers A, Kesavadas C, & Lemieux L. (2009). Evaluation of atlas-based segmentation of hippocampi in healthy humans. Magnetic Resonance Imaging, 27(8), 1104–1109. doi: 10.1016/j.mri.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, & McClarty B. (1997). Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine, 27(4), 951–959. doi: 10.1017/S0033291797005242 [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, & Pynoos RS (2004). The University of California at Los Angeles post-traumatic stress disorder reaction index. Current Psychiatry Reports, 6(2), 96–100. doi: 10.1007/s11920-004-0048-2 [DOI] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, … Ressler KJ (2014). PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 3158–3163. doi: 10.1073/pnas.1318954111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, & Jovanovic T. (2016). Childhood Maltreatment Predicts Reduced Inhibition-Related Activity in the Rostral Anterior Cingulate in Ptsd, but Not Trauma-Exposed Controls. Depress Anxiety, 33(7), 614–622. doi: 10.1002/da.22506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, & Ressler KJ (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res, 47(10), 1469–1478. doi: 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, & Barch DM (2014). Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. Journal of the American Academy of Child and Adolescent Psychiatry, 53(7), 800–813.e810. doi: 10.1016/j.jaac.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, & Gunnar MR (2006). Child maltreatment and the developing HPA axis. Horm Behav, 50(4), 632–639. doi: 10.1016/j.yhbeh.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, … Vitaliano GD (2018). Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage, 169, 443–452. doi: 10.1016/j.neuroimage.2017.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, & Polcari A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences, 109(9), E563–E572. doi: 10.1073/pnas.1115396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17, 652. doi: 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Dev Sci, 14(2), 190–204. doi: 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, … Casey BJ (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science, 13(1), 46–61. doi: 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, Dalgleish T, van der Wee NJA, Veltman DJ, Aleman A, … Elzinga BM (2014). Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 9(12), 2026–2033. doi: 10.1093/scan/nsu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, Demenescu LR, van der Wee NJA, Veltman DJ, Aleman A, … Elzinga BM (2013). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8(4), 362–369. doi: 10.1093/scan/nss007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJ, Stevens JS, Ely TD, Fani N, Smith AK, Kerley KA, … Jovanovic T. (2016). Childhood Trauma and COMT Genotype Interact to Increase Hippocampal Activation in Resilient Individuals. Front Psychiatry, 7, 156. doi: 10.3389/fpsyt.2016.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, … Jovanovic T. (2018). The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biological Psychiatry, 82(2), 106–115. doi: 10.1016/j.biopsych.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, … Bremner JD (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. The American journal of psychiatry, 159(12), 2072–2080. doi: 10.1176/appi.ajp.159.12.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, DuMont K, & Czaja SJ (2007). A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry, 64(1), 49–56. doi: 10.1001/archpsyc.64.1.49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.