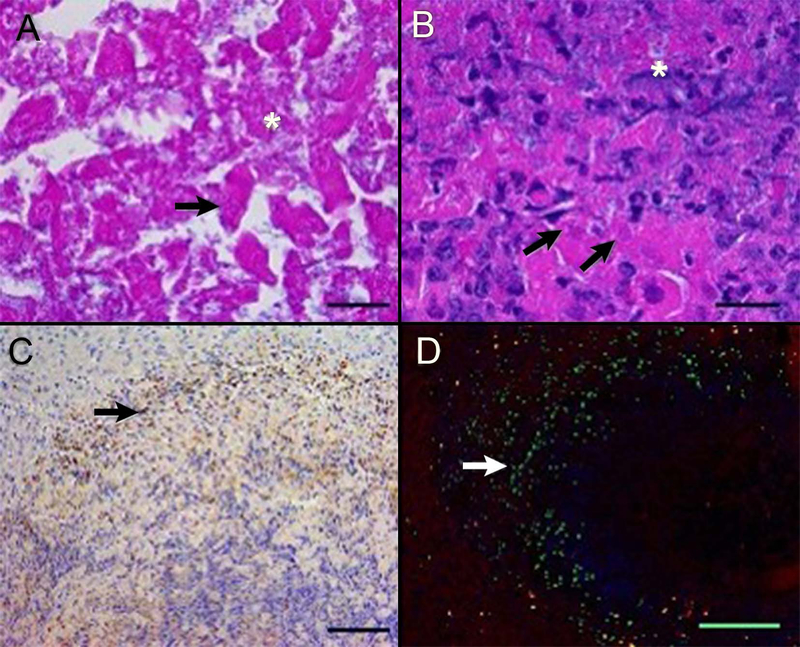
FIGURE 4.
Histology, immunohistochemistry, and immunofluorescence demonstrating aspects of cell death in thermoembolization. There is lethal and sublethal cell injury and the procedure incited an acute neutrophilic response and apoptosis within 24 hours. In the central region (panel A), hepatic cords are disrupted and cytoplasmic and nuclear detail is lost, with cell lysis (asterisk) and loss of nuclear basophilia, consistent with degradation of nucleic acids (arrow). More distant from the embolized hepatic artery (panel B), severely affected hepatocytes are lysed, with release of cytoplasmic and nuclear contents (asterisk) or undergo coagulative necrosis (arrows). Neutrophils infiltrate into the injured areas (panel C). At 24-hour post ablation, scattered cells (arrows) peripheral to the zones of immediate cell death undergo programmed cell death (apoptosis), demonstrating delayed and/or secondary injury from thermoembolization or inflammatory cell infiltrates, respectively. Porcine liver, hematoxylin, and eosin (A and B) and immunostaining for myeloperoxidase (C) and cleaved caspase-3 (D). In panel D, green = cleaved caspase-3, blue = nucleic acids, and red = phalloidin staining of polymerized actin of the cytoskeleton. Bar = 20 microns (A and B), 100 microns (C), and 500 microns (D)