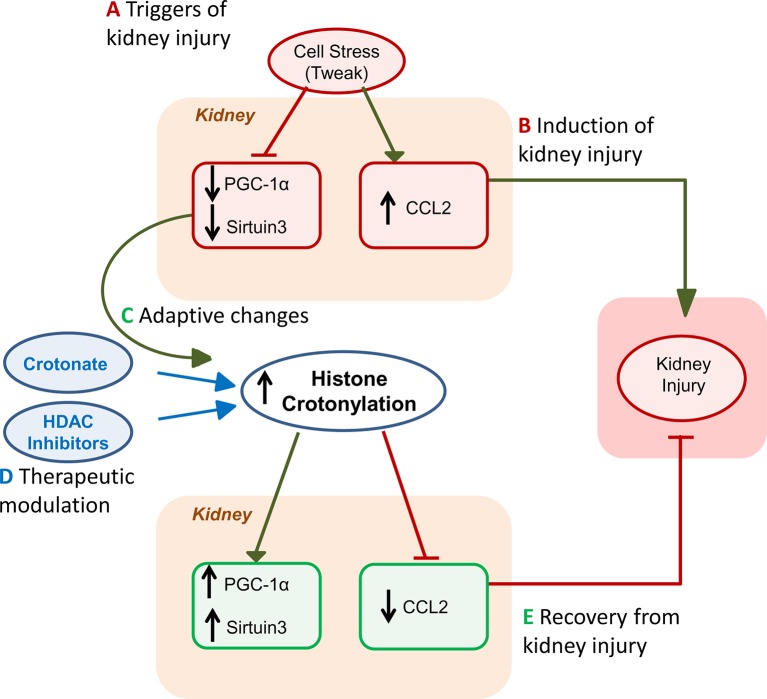
Figure 2.
Role of histone crotonylation in acute kidney injury (AKI). The figures depict the different stages of kidney injury and the integration of spontaneous histone crotonylation or therapeutic drug-induced histone crotonylation. (A) Triggers of kidney injury, such as diverse forms of cell stress, including the proinflammatory cytokine TWEAK, cause lethal or sublethal (e.g. decreased expression of the master regulator of mitochondrial biogenesis PGC1α or sirtuin 3, Sirt3) kidney cell injury and elicit a proinflammatory response (e.g. chemokine synthesis) (Morigi et al., 2015; Martin-Sanchez et al., 2018; Fontecha-Barriuso et al., 2019). (B) This will induce kidney injury that may be magnified by recruitment of inflammatory cells (Ruiz-Andres et al., 2016b). (C) During AKI, global kidney histone crotonylation increases and this may be further increased by treatment with crotonate (Ruiz-Andres et al., 2016a). Decreased expression of decrotonylases, such as Sirt3 may contribute to increased global histone crotonylation. Based on crotonate therapy results, we hypothesize that the overall impact of increased global histone crotonylation supposes a brake in the kidney injury process, and, thus, a beneficial adaptive process. (D) As already indicated, treatment with crotonate improves kidney injury and restores PGC1α or Sirt3 expression, decreasing chemokine production. Based on new knowledge of the therapeutic effects of HDAC inhibitors on kidney injury in vivo and in culture (Chen et al., 2018; Fernandez-Fernandez et al., 2018), we now hypothesize that part of this beneficial effect of HDAC inhibitors may be related to their role in crotonylation regulation, rather than or in addition to inhibition of deacetylase activity. (E) Eventually, either spontaneously or following acceleration of recovery by therapy, kidney function recovers.