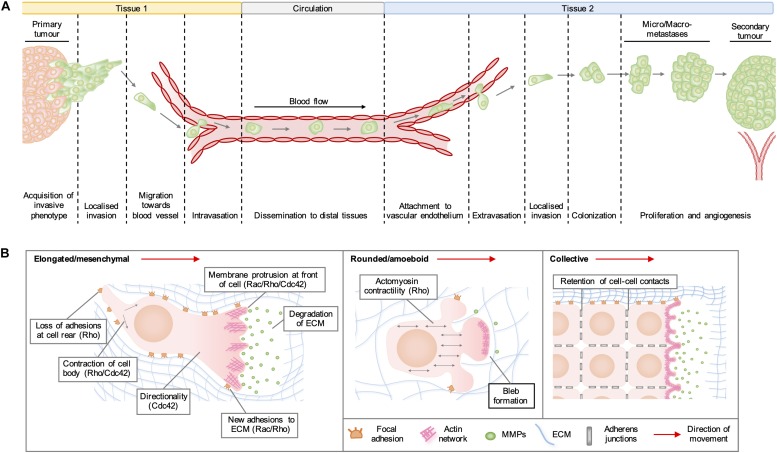
FIGURE 1.
(A) Stages of cancer metastasis. A subset of cancer cells in the primary tumor acquire an invasive phenotype and spread into the surrounding stroma, either collectively or as single cells. Some invading cells migrate toward the tumor neovasculature and enter the blood stream by migrating through vascular endothelial cell junctions in a process known as intravasation. These cancer cells can be transported by the circulation to distal tissues, where they enter narrower vessels that permit their attachment to vascular endothelial cells. Following attachment, cancer cells commonly extravasate as single cells by migrating through endothelial cell junctions and then invade into the stroma of the secondary organ. These cells may form a metastatic niche if supported by survival and growth signals in the new micro-environment. Further cell proliferation will give rise to micro- and macro-metastases and a secondary tumor is established by the formation of a new blood supply through neo-angiogenesis. (B) Modes of cell migration. Elongated cell migration involves the extension of actin-rich protrusions at the front of the cell and the localized release of matrix metalloproteinases (MMPs), which degrade extracellular matrix (ECM) proteins and create space into which the cell can move. The formation of new adhesions at the front of the cell and contraction of the cell body pull the cell in the direction of movement, whilst loss of ECM adhesions at the rear allows the cell to migrate forward. During collective cell migration, neighboring cells within a tissue remain physically linked by adherens junctions. Cells at the invasive front extend actin-rich protrusions facing the direction of movement, which form new adhesions with the ECM and enable the generation of traction forces that pull neighboring cells forward. During rounded cell migration, high actomyosin contractility produces hydrostatic pressure, which leads to the formation of membrane blebs devoid of filamentous actin at the front of the migrating cell. Highly dynamic membrane blebs fill pre-existing spaces in the matrix and form only weak attachments to the ECM.