Table 2.
Influence of the organoboron derivative on the cross-coupling reaction of N-Boc-4-iodo-l-phenylalanine.
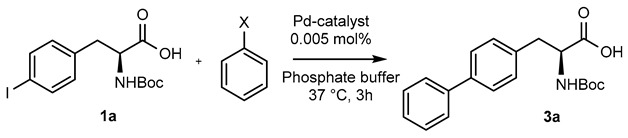
| Entry | Pd Catalyst | X | Conv (%) a |
|---|---|---|---|
| 1 | Pd-Calix-NS | B(OH)2 | 40 |
| 2 | Pd-Calix-NS | BF3-K+ | 10 |
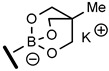
| 3 | Pd-Calix-NS |
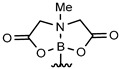
|
0 |
| 4 | Pd-Calix-NS |
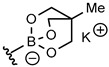
|
98 |
| 5 | Bulk Pd-Calix |
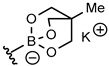
|
<5 |
| 6 | Pd-PLGA-PEG NPs |
|
97b |
| 7 |
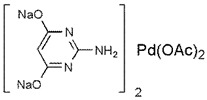
|
B(OH)2 | 95c |
aN-Boc-4-iodo-l-phenylalanine (1 equiv.), boronic acid derivatives (3 equiv.), Pd-Calix-NS (0.005 mol%), phosphate buffer (20 mM, pH = 8), 37 °C for 3 h. Conversion determined by 1H NMR analysis of the C-3 methylene signal. b 0.01% Pd, according to reference [30]. c 1 mol% Pd, according to reference [18].
