Table 4.
Reactivity of aryl and heteroaryl cyclic triolborates with N-Boc-4-iodophenylalanine.
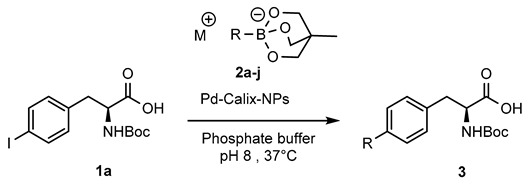
| Entry | Cyclic borate | R | Metal | pH | Product | Yield(%) a |
|---|---|---|---|---|---|---|
| 1 | 2a | Ph | K | 8.0 | 3a | 98 |
| 2 | 2a | Ph | K | 7.0 | 3a | 67 |
| 3 | 2a | Ph | K | 6.0 | 3a | 56 |
| 4 | 2b | Ph | Li | 8.0 | 3a | 30 |
| 5 | 2c | Ph | Na | 8.0 | 3a | 90 |
| 6 | 2d | Ph | Cs | 8.0 | 3a | 47 |
| 7 | 2e | Ph | TBA | 8.0 | 3a | 22 |
| 8 | 2f | p-MeOPh | K | 8.0 | 3af | 98 |
| 9 | 2g | o-Tol | K | 8.0 | 3ag | 79b |
| 10 | 2h | m-NO2Ph | K | 8.0 | 3ah | 72b |
| 11 | 2i | 2-furanyl | K | 8.0 | 3ai | 87 |
| 12 | 2j | 3-thiophenyl | K | 8.0 | 3aj | 63 |
a The reaction was conducted at 60 °C.
