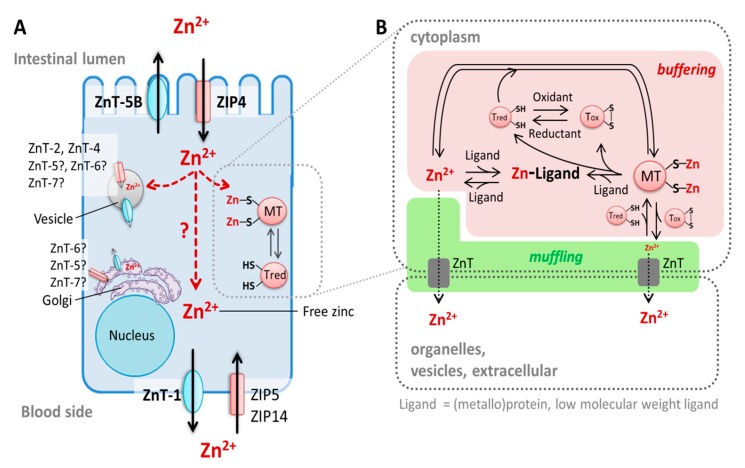
Figure 2.
Enterocyte zinc homeostasis. (A) Zinc homeostasis in enterocytes during zinc absorption. Three main zinc pools in enterocytes have been described: (i) cytoplasmic-free zinc, which is only complexed by low molecular weight ligands, (ii) protein-bound zinc, depicted here as metallothionein (MT)-bound zinc, and (iii) free zinc stored in vesicles [104]. The vesicular [102,103] and cytoplasmic-free zinc pools [101] are recognized to be involved in zinc absorption by enterocytes [105]. Cellular zinc homeostasis is maintained by three main groups of proteins: the zinc transporter (ZnT)-and the Zrt-, Irt-like protein (ZIP)-family as well as the zinc-binding metallothioneins [99]. They regulate the cytoplasmic-free zinc concentration and provide its distribution into organelles and vesicles. Exporters of zinc from vesicular stores in enterocytes remain to be identified and transfer of the divalent cation through the enterocytes after its uptake by the cells (illustrated by red arrows) is not yet fully understood. (B) Zinc buffering and muffling role of metallothioneins (MTs). MTs and other ligands (such as proteins) bind free zinc and, thereby, buffer its cytoplasmic concentration. In addition to zinc transporters, MTs represent zinc muffling moieties, which decrease free zinc content in the cytoplasm by transferring the cation to transporters, sequestering it into organelles, vesicles, or outside the cell. Notably, free zinc itself can also be transported into organelles, whereby, in this process, the ZnT solely undertakes the muffling [100]. Moreover, MTs re-distribute intracellular zinc by transferring it to other ligands, such as metalloproteins [106]. This zinc transfer may be enforced by a redox-active mechanism in which the apo-protein Thionein (Tred) binds the cation, which results in its metal-loaded form, MT, which releases zinc upon its oxidation to Thionin (Tox) (reviewed in Reference [107]).