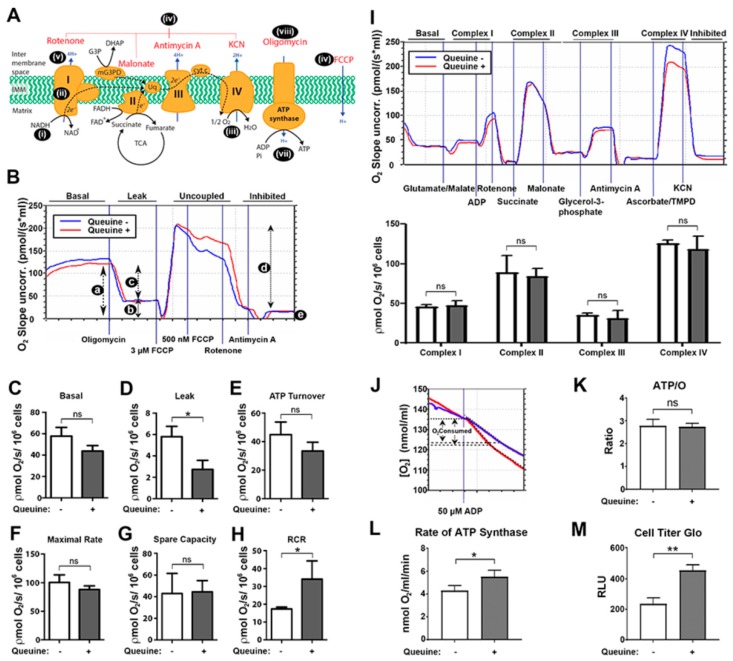
Figure 3.
Electron transport chain (ETC) activity is normal in queuine deficiency whereas leak and ATP synthesis are affected. (A) Mechanism and inhibition of the electron transport chain (ETC). The ETC (i) acquires free energy from food substrates (glucose, amino acids, fatty acids) through the intermediacy of NADH cofactor, succinate, and glycerol-3-phosphate (also fatty acids; not shown). Electrons flow stepwise (ii) through a chain of carrier complexes I-IV, situated in the mitochondrial inner membrane, with oxygen (O2) acting as the terminal electron acceptor (iii), which is reduced to water. Electron flow can be interrupted at each complex using specific inhibitors (iv), as shown. Electron movement along the ETC is coupled to proton translocation from the mitochondrial matrix into the intermembrane space (v) creating a protomotive force across the inner membrane, which can be artificially dissipated by the ionophore FCCP (vi). The flow of protons back across the membrane through the ATP synthase (complex V) drives ATP synthesis (vii) from ADP and inorganic phosphate (Pi). The activity of the ATP synthase in both forward and reverse directions can be inhibited by oligomycin (viii). (B) HeLa cells were cultured in the absence (blue lines) or presence (red lines) of 1 µM queuine for 48 h, harvested, resuspended in MiR05 buffer, and 4 × 106 cells were introduced into each chamber of an Oroboros oxygraph-2K instrument. The parameters a. basal respiration, b. oligomycin-insensitive respiration (leak; state 4o), c. oligomycin-sensitive (ATP turnover) respiration, d. maximum respiratory capacity in the presence of FCCP (state 3u), and e. the non-mitochondrial respiration rate were determined (representative trace) by the addition of the indicated agents (top figure). (C) Basal, (D) leak, (E) ATP turnover, (F) maximal/uncoupled rate, (G) spare capacity, and (H) respiratory control ratio (RCR) are graphed from repeat experiments. (I) Respirometric analysis of the ETC. After endogenous respiration had stabilized, glutamate and malate were added as substrate for complex I, cells were permeabilized with digitonin and respiration stimulated with the addition of ADP (upper panel; representative trace). Subsequently, the indicated substrates and inhibitors were added to examine the activity of complexes I–IV. Data from repeat experiments were graphed (lower panel). (J) The ATP/O ratio was measured using the NADH-linked substrates glutamate and malate (representative trace). Cells were permeabilized with digitonin and adenylate kinase inhibitor Ap5A added to each chamber. Once a stable rate of respiration was established a fixed concentration of ADP (50 µM) was added, the O2 consumption recorded and the ATP/O ratio (K) and rate of ATP synthesis (L) calculated. (M) The level of cellular ATP was measured using CellTiter-Glo reagent. Graphed data represent mean (± s.d.) for triplicate samples. n = 3. *p < 0.05, **p < 0.01, ns, not-significant, t-test.