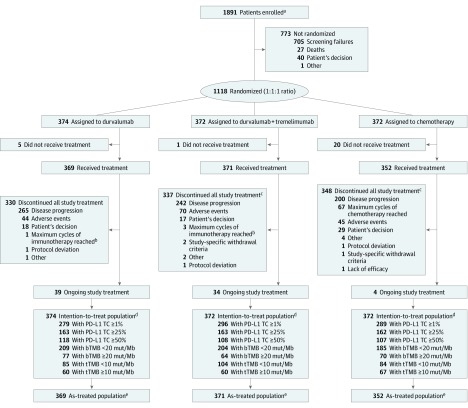
Figure 1. CONSORT Diagram.
Data cutoff date: October 4, 2018. bTMB indicates blood tumor mutational burden; mut/Mb, mutations per megabase; PD-L1, programmed cell death ligand 1; TC, tumor cell; tTMB, tissue tumor mutational burden.
aScreening consent received for PD-L1 status.
bOnly applicable for patients completing study treatment before implementation of clinical study protocol amendment, which allowed patients to continue receiving immunotherapy until disease progression, whereas previously a maximum of 12 months was allowed.
cReason for discontinuation applies to the latest component discontinued.
dIntention-to-treat population includes all randomized patients.
eAs-treated population includes all patients who received at least 1 dose of study treatment.