Abstract
More than 40 compounds have been formally licensed for clinical use as antiviral drugs, and half of these are used for the treatment of HIV infections. The others have been approved for the therapy of herpesvirus (HSV, VZV, CMV), hepadnavirus (HBV), hepacivirus (HCV) and myxovirus (influenza, RSV) infections. New compounds are in clinical development or under preclinical evaluation, and, again, half of these are targeting HIV infections. Yet, quite a number of important viral pathogens (i.e. HPV, HCV, hemorrhagic fever viruses) remain in need of effective and/or improved antiviral therapies.
I. Introduction
There are at present a forty some antiviral drugs that have been formally licensed for clinical in the treatment of viral infections.44 These are mainly used in the treatment of infections caused by human immunodeficiency virus (HIV), hepatitis B virus (HBV), herpesviruses [herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV)], orthomyxoviruses (influenza), paramyxoviruses [respiratory syncytial virus (RSV)], and hepaciviruses [hepatitis C virus (HCV)]. As these are the viruses that are most in demand of antiviral therapy, they have prompted the search for new antiviral strategies and drugs directed towards either the same molecular targets as the approved antiviral drugs or to other targets.
Most of the newly described antiviral compounds (that are currently in development) are targeted at HIV, HBV or HCV. Some are targeted at HSV, VZV or CMV, but, there are, in addition, many other important viral pathogens for which medical intervention, either prophylactic or therapeutic, is highly needed, and, these are, among the DNA viruses, the papillomaviruses [human papilloma virus (HPV)], adenoviruses, poxviruses (variola, vaccinia, monkeypox, …) and the herpesviruses Epstein–Barr (EBV) and human herpesvirus type 6 (HHV-6), and, among the RNA viruses, enteroviruses (i.e. Coxsackie B and Echo), coronaviruses [i.e. severe acute respiratory syndrome (SARS)-associated coronavirus], flaviviruses (i.e. Dengue, Yellow fever) and other RNA viruses associated with hemorrhagic fever [arenaviruses (i.e. Lassa fever), bunyaviruses (i.e. Rift Valley fever, Crimean-Congo fever) and filoviruses (i.e. Ebola and Marburg)].
Here I will describe, for each viral family, (i) which are the antiviral drugs that have been formally approved, (ii) which are the compounds that are under clinical development and thus may be considered as antiviral drug candidates, and (iii) which compounds are in the preclinical stage of development and still have a long route ahead before they could qualify as antiviral drugs (Table 1 , Figure 1 ). The virus families to be addressed are the following: parvo-, polyoma-, papilloma-, adeno-, herpes-, pox-, picorna-, flavi-, corona-, orthomyxo-, paramyxo-, arena-, bunya-, rhabdo-, filo-, reo-, and retroviruses.
Table 1.
The past, present and future of antiviral drugs (Part I: DNA viruses and retroviruses)
| Virus | Compound |
||
|---|---|---|---|
| Approved for medical use | In clinical development | In preclinical evaluation | |
| Parvo (B19) | – | – | – |
| Polyoma (JC, BK) | Cidofovir (“off label”) | – | – |
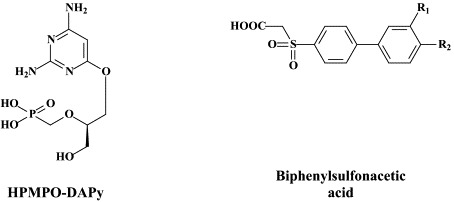
| Papillomas (HPV) | Cidofovir (“off label”) | – | cPr PMEDAP and other acyclic nucleoside phosphonates Biphenylsulfonacetic acid derivatives |
| Adeno | Cidofovir (“off label”) | – | HPMPO-DAPy |
| HDP-HPMPA, ODE-HPMPA | |||
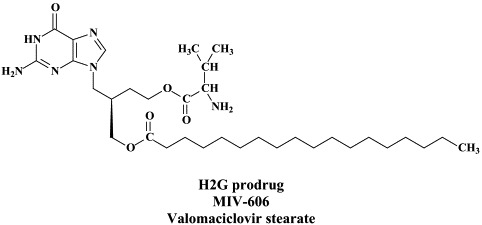
| α-Herpes (HSV-1, HSV-2, VZV) | Acyclovir | H2G prodrug | Tricyclic acyclovir derivatives |
| Valaciclovir | A-5021 | ||
| Penciclovir (topical) | Synguanol | ||
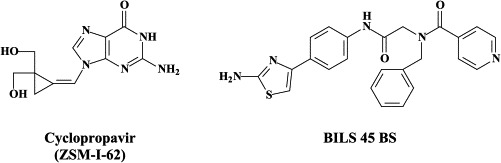
| Famciclovir | Cyclopropavir (ZSM-I-62) | ||
| Brivudin | BILS 45 BS | ||
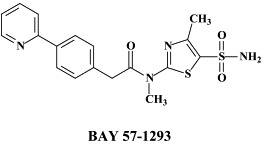
| Idoxuridine (topical) | BAY 57-1293 | ||
| Trifluridine (topical) | BCNA Cf 1742 | ||
| BCNA Cf 1743 | |||
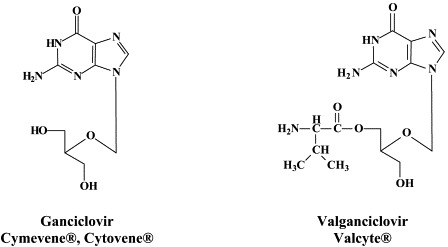
| β-Herpes (CMV, HHV-6, HHV-7) | Ganciclovir | Maribavir | CMV423 |
| Valganciclovir | HDP-CDV (CMX001) | ||
| Cidofovir | ODE-CDV | ||
| Foscarnet | 3-Deaza-HPMPA | ||
| Fomivirsen | |||
| γ-Herpes (EBV, HHV-8) | Cidofovir (“off label”) | – | North-methanocarbathymidine (N-MCT) |
| Pox (Variola, Vaccinia, Monkeypox, | Cidofovir (“off label) | – | HPMPO-DAPy |
| Molluscum contagiosum, orf, …) | HDP-CDV, ODE-CDV | ||
| HDP-HPMPA, ODE-HPMPA | |||
| CI-1033 | |||
| ST-246 | |||
| 5-X-dUrds | |||
| Pyrazolone-pyrimidine 2′-deoxy-nucleoside chimera Cyclopentenyl 1,2,3-triazole-4-carboxamide | |||
| Hepadna (HBV) | Lamivudine | Valtorcitabine | -Fluoro-,-dideoxyguanosine |
| Adefovir dipivoxil | Clevudine | Helioxanthin | |
| Entecavir | |||
| Pegylated interferon-α | |||
| Telbivudine | |||
| Retro (HIV) | Zidovudine | BMS-378806 | Cyclotriazadisulfonamide |
| Didanosine | BMS-488043 | Cyanovirin N | |
| Zalcitabine | AMD-3100 | KRH-1636 | |
| Stavudine | SCH-C | TAK-779 | |
| Lamivudine | Vicriviroc | TAK-220 | |
| Abacavir | Aplaviroc | TAK-652 | |
| Emtricitabine | Maraviroc | MIV-210 | |
| Tenofovir disoproxil fumarate | Racivir | DOT | |
| Nevirapine | AVX-754 | 4′-Ed4T | |
| Delavirdine | Reverset | PMEO-DAPy | |
| Efavirenz | Elvucitabine | PMPO-DAPy | |
| Saquinavir | Alovudine | PMDTA | |
| Ritonavir | Amdoxovir | PMDTT | |
| Indinavir | Capravirine | HDP-HPMPA, ODE-HPMPA | |
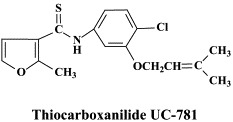
| Nelfinavir | Etravirine | Thiocarboxanilide UC-781 | |
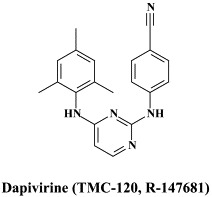
| Amprenavir | Brecanavir | Dapivirine | |
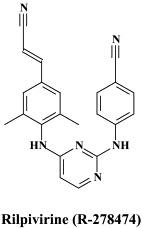
| Lopinavir | PA-457 | Rilpivirine | |
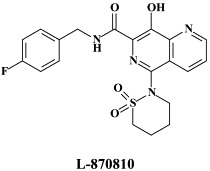
| Atazanavir | GW678248 | L-870810 | |
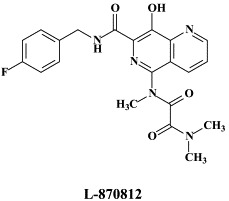
| Fosamprenavir | L-870812 | ||
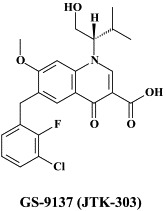
| Tipranavir | GS-9137 (JTK-303) | ||
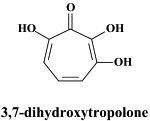
| Darunavir | Dihydroxytropolone | ||
| Enfuvirtide | Pyrimidinyl diketo acid | ||
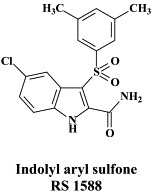
| Indolyl aryl sulfone | |||
| Pradimicin A | |||
Figure 1.





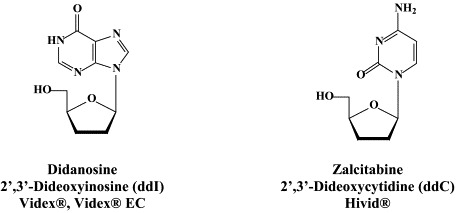
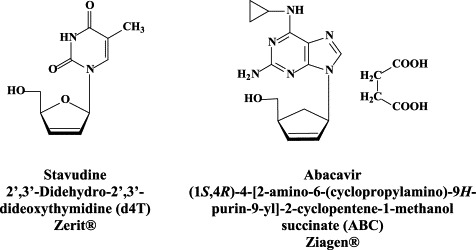
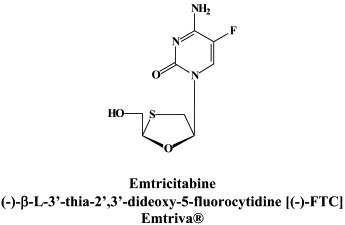
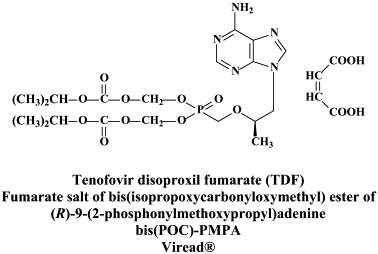
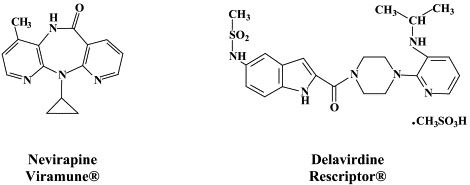
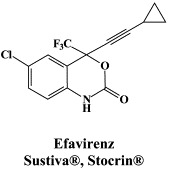


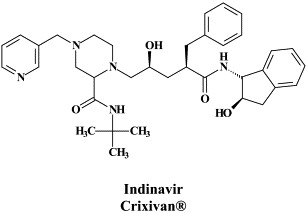
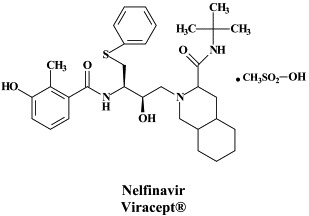

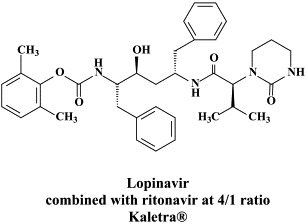
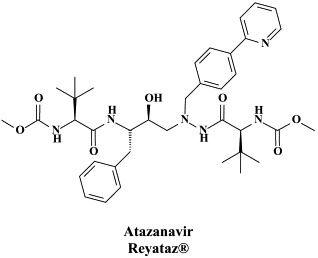
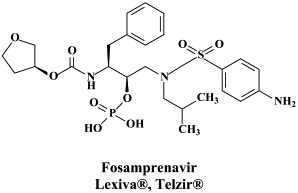
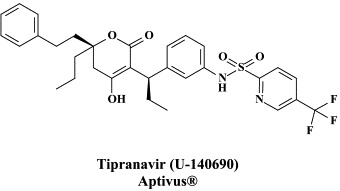
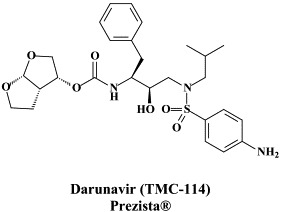
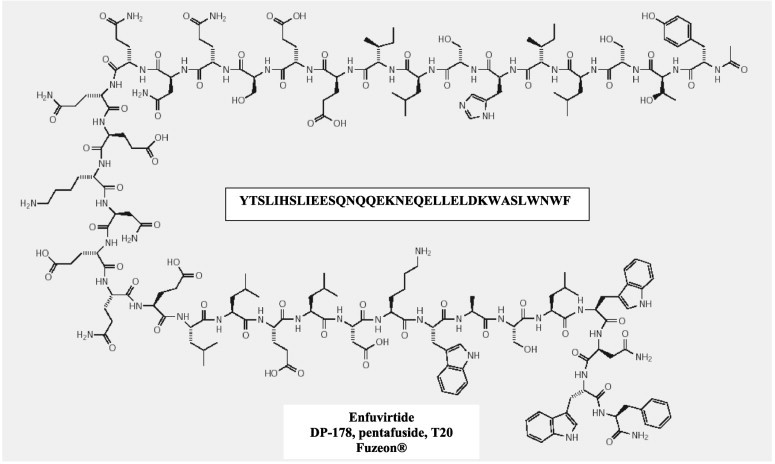

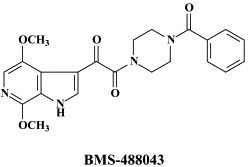
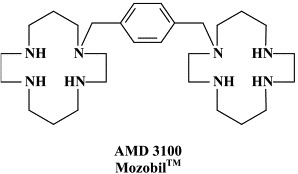
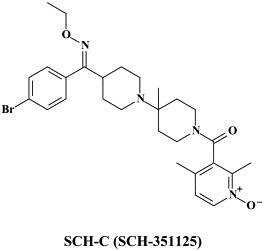
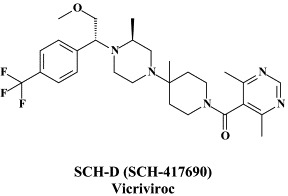
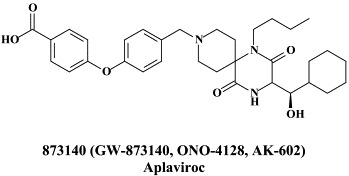
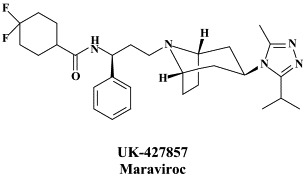
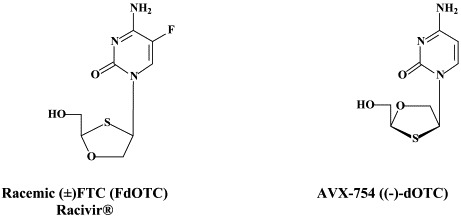
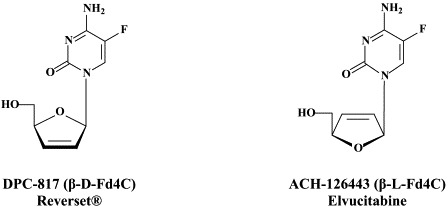
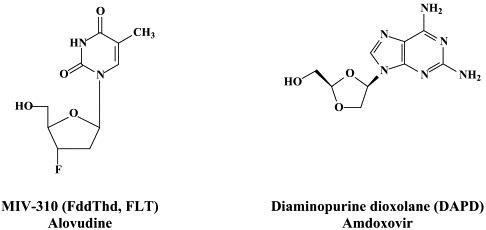

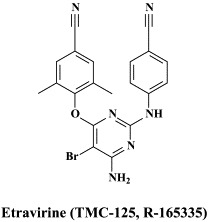


Structural formulae of antiviral compounds.
II. Parvoviruses
No significant attempts have been made to develop compounds with potential activity against B19, the only parvovirus that is pathogenic for humans and responsible for erythema infectiosum, the so-called fifth disease, in children.
III. Polyomaviruses
No antiviral drugs have been formally approved for the treatment of polyomavirus (JC and BK)-associated diseases such as progressive multifocal leukoencephalopathy (PML) and hemorrhagic cystitis in patients with AIDS. There are, however, anecdotal case reports pointing to the efficacy of cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC], which has been licensed under the trademark name Vistide® for the intravenous treatment of CMV retinitis in AIDS patients] in the treatment of polyoma (JC and BK) virus infections, particularly PML, in AIDS patients.39
The in vitro activity of various acyclic nucleoside phosphonates, among which HPMPC (cidofovir), against murine and primate polyomaviruses has been well established.1 Esterification of cidofovir (CDV) with hexadecyloxypropyl (HDP) or octadecyloxyethyl (ODE) groups, as in HDP-CDV or ODE-CDV, respectively, resulted in up to a 3-log decrease of the 50% effective concentration (EC50) and up to 2-log increase of the selectivity index.130
IV. Papillomaviruses
As for polyomaviruses, no antivirals have been licensed for the treatment of human papillomavirus (HPV)-associated diseases, including warts (verruca vulgaris), condyloma acuminatum, papillomatosis (i.e. recurrent respiratory papillomatosis), and cervical, vulvar, penile and (peri)anal dysplasia (evolving to carcinoma). Various strategies, including surgery and other destructive therapies, antiproliferative agents, and immunotherapies have been used for the treatment of HPV-associated lesions.141 Cidofovir has been used “off label”, with success, in the topical and, occasionally, systemic treatment of HPV-associated papillomatous lesions.39 In many instances, a virtually complete and durable resolution of the lesions was achieved following topical application of cidofovir as a 1% gel or cream.
In addition to cidofovir, other acyclic nucleoside phosphonates, such as cPrPMEDAP [N6-cyclopropyl-9-(2-phosphonylmethoxyethyl)-2,6-diaminopurine], are being explored for their potential in the treatment of HPV-associated papillomas and dysplasias.39., 51. These compounds have been shown to specifically induce apoptosis in HPV-infected cells, which, in turn, may be related to their ability to restore the function of the tumor suppressor proteins p53 and pRb (which are neutralized by the oncoproteins E6 and E7, respectively, in HPV-infected cells).
Recently, biphenylsulfonacetic acid derivatives have been described as inhibitors of HPV E1 helicase-associated ATP hydrolysis.60., 155. Although these novel ATPase inhibitors can hardly be considered to be good drug candidates, they may serve as leads for further optimization as potential antiviral agents active against multiple HPV types.155
As for so many other virus infections (see supra), RNA interference (RNAi), based on small interfering RNAs, has been advocated to block HPV infections (HPV16 E6 oncogene expression).118., 148. This siRNA approach could be particularly useful for silencing HPV oncogenes, as in cervical intraepithelial neoplasia.
V. Adenoviruses
For the treatment of adenovirus infections, which could be quite severe in immunocompromised patients (i.e. allogeneic hematopoietic stem-cell transplant recipients), no antiviral drugs have been officially approved. Anecdotal reports have pointed to the efficacy of cidofovir against adenovirus infections in such patients.39 Among the novel compounds that could be further explored for the treatment of adenovirus infections are (S)-2,4-diamino-6-[3-hydroxy-2-phosphonomethoxy)propoxy]pyrimidine [HPMPO-DAPy],51 which akin to some “older” compounds like (S)-9-(3-hydroxy-2-phosphono-methoxypropyl)adenine (HPMPA), the N7-substituted acyclic nucleoside 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine S-2242, the 2′,3′-dideoxynucleosides zalcitabine (ddC) and alovudine (FddT, FLT) have been found to inhibit adenovirus replication in vitro.113
In fact, ddC was also found effective in vivo, in a mouse model for adenovirus pneumonia.111 Also, ether lipid-ester [hexadecyloxypropyl (HDP) and octadecyloxyethylI (ODE)] prodrugs of HPMPC and HPMPAI have been designed that inhibit adenovirus replication in vitro at significantly lower concentrations than the parent compounds.73
VI. α-Herpesviruses (HSV-1, HSV-2, VZV)
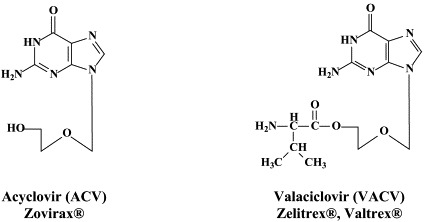
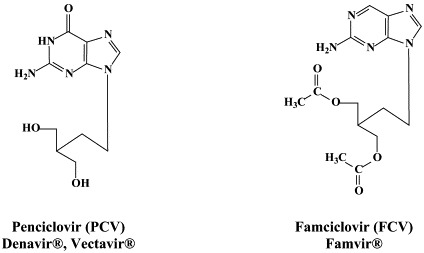
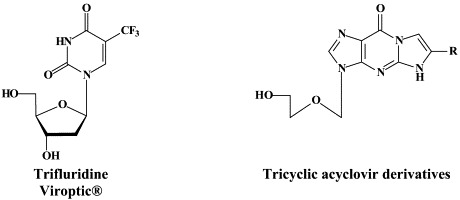
For the treatment of HSV-1, HSV-2 and VZV, a number of compounds have been approved: acyclovir and its oral prodrug valaciclovir; penciclovir and its oral prodrug, famciclovir; idoxuridine, trifluridine and brivudin. Penciclovir, idoxuridine and trifluridine are used topically, primarily in the treatment of herpes labialis (penciclovir) and herpetic keratitis (idoxuridine, trifluridine). Acyclovir can be used orally, intravenously or topically, whereas valaciclovir and famciclovir are administered orally, in the treatment of both HSV and VZV infections. Brivudin (available in some European countries) is used orally for the treatment of herpes zoster, but is also effective against HSV-1 infections.
While acyclovir (and its oral prodrug valaciclovir) have remained the gold standard for the treatment of HSV and VZV infections, few attempts have been made to bring other anti-HSV (or anti-VZV) agents into the clinic, with the exception of the H2G prodrug, which, since quite a number of years, is still in clinical development for the treatment of herpes zoster.46 From acyclovir (ACV) and ganciclovir (GCV) tricyclic derivatives, i.e. tricyclic acyclovir (TACV), and 6-substituted derivatives thereof which have similar (or only slightly decreased) antiviral potency but increased lipophilicity as compared to the parent compounds (ACV and GCV), and, in addition, show interesting fluorescence properties.68
Worth considering for clinical development as anti-HSV (and anti-VZV) agents are a number of carbocyclic guanosine analogues, such as A-5021, cyclohexenylguanine, and the methylene cyclopropane synguanol.49 All these compounds owe their selective antiviral activity to a specific phosphorylation by the HSV- or VZV-encoded thymidine kinase (TK); upon phosphorylation to their triphosphate form, they act as chain terminators in the DNA polymerization reaction. In the (rare) circumstances that HSV or VZV becomes resistant to the acyclic (or carbocyclic) guanosine analogues due to TK deficiency (TK−), the pyrophosphate analogue foscarnet could be useful to treat TK− HSV or TK− VZV infections (in immunocompromised patients).
Recently, a second generation of methylene cyclopropane analogues, the 2,2-bishydroxymethyl derivatives, has been synthesized.166 These compounds may have potential, not only for the treatment of HSV-1, HSV-2 and VZV, but also β-herpes (CMV, HHV-6, HHV-7) and γ-herpes (EBV, HHV-8) infections.85 In particular, ZSM-I-62 (Cyclopropavir) has been reported to be very effective in reducing mortality of mice infected with murine CMV.84 Of a recently synthesized series of 9-{[3-fluoro-2-(hydroxymethyl)cyclopropylidene]methyl}adenines and guanines, the (Z)-{[trans-(3-fluoro-2-hydroxymethyl)cyclopropylidene]methyl}adenine was quoted as being effective against EBV at an EC50 < 0.03 μM.167
New anti-HSV agents targeting the viral helicase–primase complex, the thiazolylphenyl derivatives BILS 179 BS and BAY 57-1293, were recently reported to have in vivo efficacy in animal models of HSV-1 and HSV-2 infections.30., 87. These compounds seem to function by diminishing the affinity of the helicase–primase complex for the HSV DNA. The heterotrimeric helicase–primase complex is composed of the UL5, UL8 and UL52 gene products with DNA helicase DNA-dependent ATPase and DNA primase activity.29 Resistance to aminothiazolylphenyl-based inhibitors has been shown to arise from single amino acid changes in the UL5 protein.103
The antiviral potency of BAY 57-1293 was reported to be superior to all compounds that are currently used to treat HSV infections.13 In recent studies, BAY 57-1293 was shown to be more efficacious than famciclovir in the therapy of HSV-1 infections in BALB/C mice.16 Also, BILS 45BS, which is structurally related to BILS 179 BS, exhibited excellent efficacy in the oral treatment of acyclovir-resistant (ACVr) HSV-1 infections in nude mice,57 highlighting the potential of this class of antiherpetic agents for the treatment of ACVr HSV disease in humans.
RNA interference (RNAi), as generated by small interfering RNAs (siRNAs), has been recently pursued as a powerful tool to silencing disease,138 including viral infections. Palliser et al.123 have shown that vaginal instillation of siRNAs targeting the HSV-2 UL27 and UL29 genes (which encode an envelope glycoprotein and a DNA binding protein, respectively) protected mice from a lethal HSV-2 infection; the siRNAs were mixed with lipid so as to ensure their uptake by the cells (vagina and ectocervix). From this study it was concluded that siRNAs may be attractive candidates for application as microbicides to prevent viral infection.123
A new class of anti-VZV compounds are the bicyclic furo (2,3-d)pyrimidine nucleoside analogues (BCNAs), represented by Cf 1742 and Cf 1743.110 These compounds are exquisitely active against VZV.40 They inhibit the replication of VZV, but not that of other viruses (including HSV), at subnanomolar concentrations, with a selectivity index in excess of 100,000.2 Given the extremely high potency and selectivity of the BCNAs they warrant to be further developed towards clinical use, i.e. against herpes zoster.
VII. β-Herpesviruses (CMV, HHV-6, HHV-7)
Five compounds have been licensed to treat CMV infections: ganciclovir, its oral prodrug valganciclovir, foscarnet, cidofovir and fomivirsen. [Foscarnet has also proven efficacious in the treatment of other DNA virus (i.e., hepatitis B) infections.72] With the exception of fomivirsen (an antisense oligonucleotide) which targets the CMV immediate-early mRNA, all other licensed anti-CMV drugs target the viral DNA polymerase. Ganciclovir must first be phosphorylated by the CMV-encoded protein kinase (the UL97 gene product) which is also the principal site for mutations engendering resistance towards this compound. Toxic side effects (i.e. bone–marrow suppression for ganciclovir, nephrotoxicity for foscarnet and cidofovir) have prompted the search for new inhibitors of CMV.41
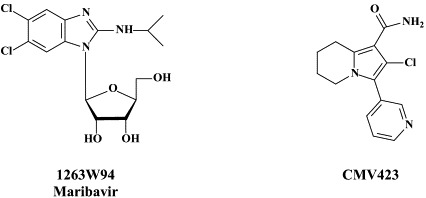
Several benzimidazole ribonucleosides, among which maribavir (previously also known as 1263W94), have been accredited with specific activity against human CMV. Maribavir seems to target the UL97 protein kinase,15 and, as the UL97 gene product has been shown to account for the release of CMV nucleocapsids from the nucleus,90 maribavir may be assumed to target a stage in the viral life cycle that follows viral DNA maturation and packaging. Preclinical pharmacokinetic and toxicological studies have shown that maribavir has a favorable safety profile and excellent oral bioavailability.89
Phase I/II dose-escalation trials in HIV-infected men with asymptomatic CMV shedding further indicated that maribavir is rapidly absorbed following oral dosing and reduces CMV titers in semen.93 Maribavir is currently in a prophylaxis study in allogenic stem cell transplant recipients with results expected in 2006 [to be divulged by ViroPharma Inc., according to Biron14]. Biron14 also mentioned two other compounds, i.e. BAY 38-4766 and GW275175X, which entered clinical development but were then not further developed despite a favorable safety profile (BAY 38-4766) or shelved in favor of the advancement of maribavir (GW275175X).
While maribavir is primarily active against CMV, 2-chloro-3-pyridin-3-yl-5,6,7,8-tetrahydroindolizine-1-carboxamide (CMV423) has potent and selective in vitro activity against all three human β-herpesviruses, CMV, HHV-6 and HHV-7.35., 36. As compared to ganciclovir and foscarnet, CMV423 has higher antiviral potency and lower cytotoxicity. It is targeted at an early stage of the viral replication cycle (following viral entry but preceding viral DNA replication), which is regulated by a cellular process that may involve protein tyrosine kinase activity.
Some cellular kinase inhibitors have been found to enhance the antiviral activity of maribavir.26 It may be useful, therefore, to further explore the possibility of therapeutically useful combinations of maribavir and cellular kinase inhibitors such as CMV423. Combination of maribavir with ganciclovir should not be recommended, since maribavir antagonizes the antiviral action of ganciclovir.25
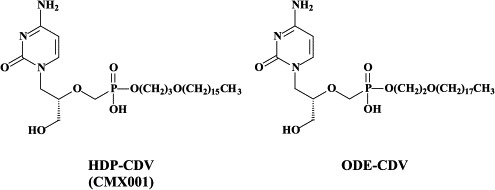
Alkoxyalkyl esters of cidofovir (i.e. HDP-CDV) have been developed that retain the efficacy of the parent compound,10 without the associated renal toxicity28 and with significantly improved oral bioavailabilities. HDP-CDV (CMX001) is under current development as an oral drug for the treatment of poxvirus infections as well as CMV and other herpesvirus infections.
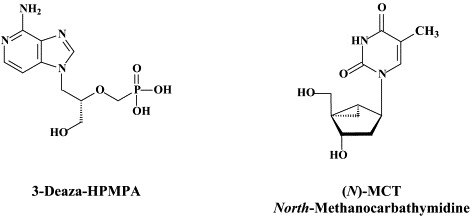
There is, at present, no standardized antiviral treatment for HHV-6 infections and also their potential clinical indications remain ill-defined. From a comparative study, A-5021, foscarnet, S2242, and cidofovir emerged as the most potent compounds with the highest antiviral selectivity against HHV-6.50 The latter three also proved to be the most potent against HHV-7.50 In addition to cidofovir, HPMPA and its 3-deaza analogue 3-deaza-HPMPA have also been identified as potent and selective inhibitors of HHV-6 replication.112
However, the most promising anti-HHV-6 activity was demonstrated by CMV423, a compound that has been shown previously to be highly effective in vitro against CMV.142 The compound exhibited a potency (EC50: 0.02–0.05 μM) and selectivity (SI > 2000) against HHV-6(A), which by far exceeded that of the standard anti-herpesvirus agents acyclovir, ganciclovir, foscarnet and cidofovir.47 The in vitro antiviral actionprofile of CMV423 is such that it deserves to further explored for its in vivo potential in the treatment of CMV and HHV-6(A) infections.
VIII. γ-Herpesviruses (EBV, HHV-8)
Although a number of the aforementioned approved anti-herpetic drugs, such as acyclovir, ganciclovir, brivudin and cidofovir, have proven to be effective against the in vitro replication of EBV and HHV-8,50 none of these (or any other) antiviral drugs have been formally approved for the treatment of diseases associated with EBV (i.e. mononucleosis infectiosa, B-cell lymphoma, lymphoproliferative syndrome, Burkitt's lymphoma, nasopharyngeal carcinoma) or HHV-8 (Kaposi's sarcoma, primary effusion lymphoma, multicentric Castleman's disease). It would seem appealing to further examine established anti-herpetic drugs, such as cidofovir, and other acyclic nucleoside phosphonates such as HPMPA, or prodrugs thereof, for their potential in the therapy of EBV- and HHV-8-associated malignancies.
Also, new nucleoside analogues, such as the conformationally locked nucleoside analogue north-methanocarbathymidine [(N)-MCT],169 which have been previously134 shown to block the replication of HSV-1 and HSV-2, should be further explored for their potential in the prevention and treatment of HHV-8-associated malignancies: in casu, (N)-MCT, which is specifically triphosphorylated in HHV-8-infected cells undergoing lytic replication efficiently blocks HHV-8 DNA replication in these cells.169 In fact, the antiviral activity spectrum of (N)-MCT not only includes γ-herpesviruses (EBV, HHV-8) and α-herpesviruses (HSV-1, HSV-2, VZV) but also poxviruses. (N)-MCT would be activated by the viral thymidine kinase (TK) homologs and inhibit the viral DNA polymerase. The compound has been demonstrated to be effective in vivo in reducing the mortality of mice infected with HSV-1 or orthopoxviruses.125
IX. Poxviruses (variola, vaccinia, monkeypox, molluscum contagiosum, orf …)
Thiosemicarbazenes, i.e. isatin-β-thiosemicarbazone and N-methyl-isatin-β-thiosemicarbazone (marboran or methisazone) were investigated in the 1960s for their efficacy against orthopoxviruses. They have had a lengthy history as prophylactic therapeutics with potential efficacy against Mycobacterium tuberculosis. However, it has become recently clear that this class of compounds has little, if any, potential for orthopoxvirus infections (i.e. cowpox virus, a surrogate virus for variola virus).129
Several nucleoside analogues (i.e. S2242, 8-methyladenosine, idoxuridine) and nucleotide analogues (i.e. cidofovir, HPMPO-DAPy,) have proven to be effective in various animal models of poxvirus infections.48 In particular, cidofovir has shown high efficacy, even after administration of a single systemic (intraperitoneal) or intranasal (aerosolized) dose, in protecting mice from a lethal respiratory infection with either vaccinia or cowpox. Cidofovir has demonstrated high effectiveness in the treatment of disseminated progressive vaccinia in athymic-nude mice.115
In humans, cidofovir has been used successfully, by both the topical and intravenous route, in the treatment of orf and recalcitrant molluscum contagiosum in immunocompromised patients.38 Cidofovir (HPMPC) and its congeners (HPMPDAP and HPMPO-DAPy) are highly effective against orf in human and ovine cell monolayers and organotypic ovine raft cultures.31 Given the in vitro activity of cidofovir against variola (smallpox) and other poxviruses, and the in vivo efficacy of cidofovir against various poxvirus infections in animal models and humans, it can be reasonably assumed that cidofovir should be effective in the therapy and/or prophylaxis of smallpox in case of an inadvertent outbreak or biological attack with the variola virus.
Being a phosphonate analogue, cidofovir only has limited oral bioavailability. In case of an outbreak of smallpox, it would be useful to have an orally active drug at hand.122 To this end, hexadecyloxypropyl-cidofovir (HDP-CDV) and octadecyloxyethyl-cidofovir (ODE-CDV) were designed as potential oral prodrugs of cidofovir. These alkyloxyalkyl esters of cidofovir were found to significantly enhance inhibition of the replication of orthopoxviruses (i.e. vaccinia, cowpox) in vitro.83 HDP-CDV and ODE-CDV given orally were as effective as cidofovir given parenterally for the treatment of vaccinia and cowpox infections.128
HDP-CDV has proven effective in the treatment of a lethal vaccinia virus respiratory infection in mice.140 Furthermore, HDP-CDV and ODE-CDV, when given orally, proved highly efficacious in a lethal (aerosol) mousepox (ectromelia) virus model,19 further attesting as to the potential usefulness of the alkyloxyalkyl esters of cidofovir in the oral therapy and prophylaxis of poxvirus infections. This potential usefulness has been recently extended to the alkoxyalkyl esters of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine (HPMPA) for the treatment of orthopoxvirus (i.e. vaccinia, cowpox) as well as cytomegalovirus infections.11., 95.
In fact, as indicated by the most recent findings with cidofovir in mice infected with ectromelia (mousepox) virus encoding interleukin-4,132 and monkeys infected with monkeypox,145 cidofovir (CDV) (and HDP-CDV and/or ODE-CDV) still provide the best current hope for effective control of virulent poxvirus infections.
Mutations in the E9L polymerase gene, i.e. A314T and A684V, of vaccinia virus have been associated with cidofovir resistance.3 Cidofovir resistance was associated with diminished virulence and reduced fitness in vivo, in mice.3 Cidofovir (CDV) still protected mice against CDV-resistant vaccinia virus.3., 88.
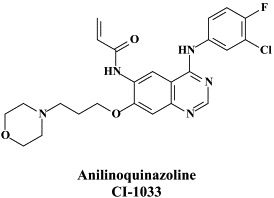
In addition to the nucleotide analogue cidofovir, which primarily acts as a viral DNA chain terminator (for vaccinia virus DNA polymerase after it has been incorporated at the penultimate position),105 antiviral strategies for poxvirus infections may also be based on inhibitors of cellular processes, i.e. signal transduction pathways. In this respect, the 4-anilinoquinazoline CI-1033, an ErbB tyrosine kinase inhibitor, was found to block variola virus replication in vitro and vaccinia virus infection in vivo.159
Likewise, Gleevec® (STI-571, Imatimib), an Abl-family kinase inhibitor used to treat chronic myelogenous leukemia in humans was shown to suppress poxviral dissemination in vivo by several orders of magnitude and to promote survival in infected mice,131 suggesting possible use for this drug in treating smallpox or complications associated with vaccination against smallpox. Because the drug targets host rather than viral molecules, it is less likely to engender resistance compared to more specific antiviral agents. Collectively,131., 159. inhibitors of host-signaling pathways exploited by poxviral pathogens may represent potential antiviral therapies.
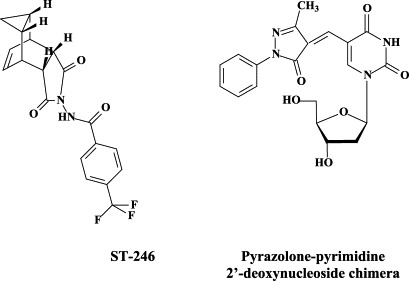
Recently, a new anti-poxvirus compound (ST-246) has been described, which is orally bioavailable, acts according to a novel mechanism of action, targeting a specific viral product (i.e. vaccinia virus F13L) required for extracellular virus particle formation and protecting mice from a lethal orthopoxvirus challenge.158 These properties make ST-246 an attractive candidate for development as a smallpox antiviral drug that could be stockpiled for use in the treatment and prevention of smallpox virus infection in the event of a bioterrorist threat.
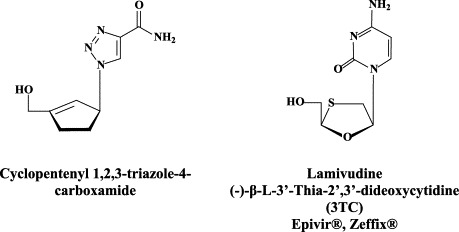
As already mentioned above, a wealth of nucleoside analogues, i.e. 5-substituted 2′-deoxyuridines (5-X-dUrds, related to idoxuridine) and neplanocin analogues, have been described as potent inhibitors of vaccinia virus [as the paradigm of poxviruses37]. Several new congeners have been recently added to this list: i.e. 5-(dimethoxymethyl)-2′-deoxyuridine,58 pyrazolone-pyrimidine-2′-deoxynucleoside chimera,59 and a cyclopentenyl 1,2,3-triazole-4-carboxamide.24 As has been demonstrated for many other viruses, small-interfering (si)RNAs have also proved effective against vaccinia virus, i.e. E3L-specific siRNAs targeting the double-stranded (ds)RNA binding protein E3L.34
X. Hepadnaviruses (HBV)
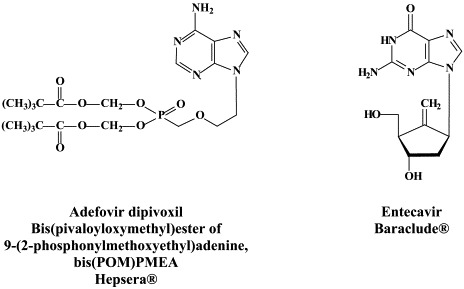
An estimated 400 million people worldwide are chronically infected with the hepadnavirus HBV; approximately 1 million die each year from complications of infection, including cirrhosis, hepatocellular carcinoma, and end-stage liver disease. Formally approved for the treatment of chronic hepatitis B are lamivudine, adefovir dipivoxil, (pegylated) interferon-α2 and entecavir. Whereas lamivudine, adefovir and entecavir [and other nucleoside analogues which are still in (pre)clinical development] act as genuine antiviral agents at the HBV-associated reverse transcriptase, interferon, in the chronic hepatitis B setting, primarily acts as an immunomodulator.
Pegylated interferon-α2b is effective in the treatment of HBeAg-positive chronic hepatitis B, but no additional benefit is achieved if it is combined with lamivudine.81 Nor does the addition of ribavirin seem to increase the efficacy of interferon in the treatment of HBeAg-positive chronic hepatitis B.101 Combination of pegylated interferon α-2b with adefovir dipivoxil, however, led to a marked decrease in serum HBV DNA and intrahepatic covalently closed circular DNA (cccDNA); which was significantly correlated with HBsAg reduction in patients with chronic hepatitis B.157
Whereas interferon therapy, also because of its unavoidable side effects (influenza-like symptoms) is not recommended for a duration longer than 1 year, the nucleos(t)ide analogues can, in principle, be administered for quite a number of years. For lamivudine (3TC), however, this prolonged treatment if compounded by the emergence of both virological and clinical resistance at an accumulating rate of approximately 20 percent of the patients per year (70% after 4 years of treatment). This resistance is primarily due to the emergence of the rt M204 I/V mutation in the YMDD motif of the HBV DNA polymerase [although, as has recently been demonstrated, lamivudine-resistant mutations can also emerge outside the YMDD motif].161
Resistance to adefovir dipivoxil may also emerge, but less frequently: not more than 6 percent after 3 years,71 although it may rise to 18 percent after 4 years106 and 29% of patients after 5 years of therapy [as cited by Osborn and Lok].120 Adefovir dipivoxil is the oral prodrug of adefovir [PMEA, 9-(2-phosphonylmethoxyethyl)adenine], which, after intracellular conversion to the diphosphate form, acts as a competitive inhibitor or alternative substrate for the HBV reverse transcriptase, and, when incorporated into the DNA, acts as a chain terminator, thereby preventing DNA chain elongation.42
In patients with chronic HBV infection who were either positive107 or negative70 for hepatitis B e-antigen, 48 weeks of treatment with a dose of adefovir dipivoxil as low as 10 mg per day resulted in significant improvement of all parameters of the disease (histological liver abnormalities, serum HBV DNA titers and serum alanine aminotransferase levels). In patients with HBeAg-negative chronic hepatitis B, the benefits achieved from 48 weeks of adefovir dipivoxil were lost when treatment was discontinued, but maintained if treatment was continued through week 144.71 Adefovir dipivoxil (10 mg daily) treatment over 52 weeks proved safe and effective in Chinese subjects with HBeAg-positive chronic hepatitis B and during this period did not lead to emergence of drug resistance.165
Resistance to adefovir dipivoxil is associated with the rt N236T and rt A181V/T mutations,121 as demonstrated in samples from patients with chronic HBV infection. Emergence of the rt A181V/T and rt N236T mutations is more common in lamivudine-resistant patients than in treatment-naïve patients.98 Adefovir resistance can be associated with viral rebound, hepatitis flares and hepatic decompensation.64 It has been suggested to combine lamivudine with adefovir dipivoxil, even in patients with lamivudine-resistant HBV, so as to prevent emergence of adefovir resistance65 In fact, adefovir dipivoxil should be added, i.e. in HBeAg-negative patients, to lamivudine as soon as genotypic resistance to lamivudine has developed.94
Entecavir, one of the most recent antiviral drugs launched for clinical use, has in vitro and in vivo potency that seems to be greater than that of lamivudine: in patients with chronic hepatitis B infection it has proven efficacious at a dose as low as 0.5 mg per day.91 The active (triphosphate) metabolite of entecavir would accumulate intracellularly at concentrations that are inhibitory to 3TC-resistant HBV DNA polymerase.99 It is not clear, however, how this translates to clinical efficacy of entecavir against lamivudine-resistant HBV infections. Early studies of entecavir indicate a low resistance potential, but resistance development over time must await the results of ongoing clinical trials.108
Comprehensive studies with entecavir carried out in patients with either HBeAg-positive chronic hepatitis B22 or HBeAg-negative chronic hepatitis B92 pointed out that the rates of histologic improvement, virologic response, and normalization of alanine aminotransferase levels were significantly higher at 48 weeks of treatment with entacavir than with lamivudine.22., 92. No case of resistance was detected after two years of entecavir therapy in patients who had not been previously treated with lamivudine. However, 10% of those patients that had failed on lamivudine therapy developed entecavir resistance after two years of therapy.170
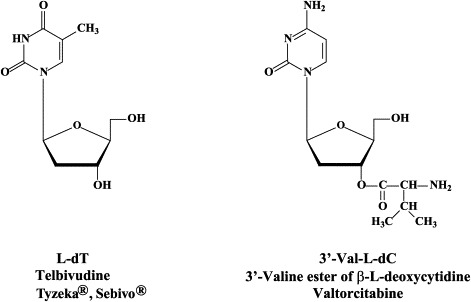
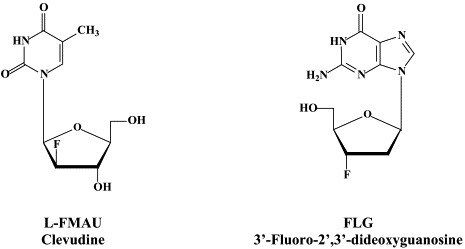
A number of L-nucleosides, i.e. β-L-thymidine (L-dT, Telbivudine), the 3′-valine ester of β-L-2′-deoxycytidine (Val-L-dC, Valtorcitabine) and 1-(2-fluoro-5-methyl-β-L-arabinosyl)uracil (L-FMAU, Clevudine) are in clinical development for the treatment of chronic hepatitis B [see Hu et al.78]. As far as the role of deoxythymidylate (dTMP) kinase in the metabolism of clevudine is concerned, clevudine showed potent antiviral activity, which was sustained for 6 months after a 12-week treatment period in HBeAg-positive chronic hepatitis B patients.96 Telbivudine did not show drug interaction with lamivudine or adefovir dipivoxil, which would allow combination of telbivudine with these drugs from a pharmacokinetics viewpoint.168
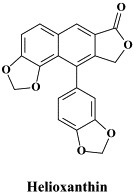
Other compounds in preclinical development include 2′,3′-dideoxy-3′-fluoroguanosine (FLG),80 racivir and L-Fd4C. These compounds are also active against HIV (see Part II). FLG proved equally effective against wild-type, lamivudine-resistant and/or adefovir-resistant HBV mutants.80 A new class of chemicals, represented by helioxanthin, has been recently described:162 these compounds would inhibit HBV replication by a mechanism of action that is different from any other anti-HBV agents described so far.23
Moreover, tenofovir disoproxil fumarate (TDF) and emtricitabine [(−)FTC, the 5-fluoro-substituted counterpart of lamivudine)], which have both been licensed, individually and in combination, for the treatment of HIV infections (AIDS), may also be considered and further pursued, individually or in combination, for use in the treatment of chronic hepatitis B. TDF has been considered an important new therapeutic tool for the induction of complete remission in patients wit lamivudine-resistant HBV infection;150 it may be a highly effective rescue drug for HBV-infected patients with diminished responsiveness to treatment with lamivudine and adefovir dipivoxil.151 At the dose used for the treatment of HIV infections, that is 300 mg/day, TDF has been found effective against wild-type and lamivudine-resistant HBV strains in HBV/HIV-coinfected patients.12
Like TDF, emtricitabine [(−)FTC] has activity against both HIV and HBV, and should, therefore, be considered for use in patients coinfected with HIV and HBV.135 An interesting recommendation has been proposed for the care of patients with chronic HBV and HIV co-infection.143 They should be put on the combination of TDF with (−)FTC, which would cover both the HBV and HIV infection. Only if no antiretroviral therapy would be used in these patients, adefovir dipivoxil and/or pegylated interferon may be installed depending on whether they are HBeAg-negative or -positive, respectively.143
As has been shown for many other viruses, the RNA interference (RNAi) approach based on short interfering RNAs (siRNAs) can also be applied to specifically inhibit HBV replication in vitro, in cell culture,124., 163. and in vivo, in mice transfected with an HBV plasmid.109 In fact, several studies have demonstrated that siRNAs are capable of specifically inhibiting HBV replication in vivo 67., 86., 109. and thus may constitute a new therapeutic strategy for HBV infection.
XI. Retroviruses (HIV)
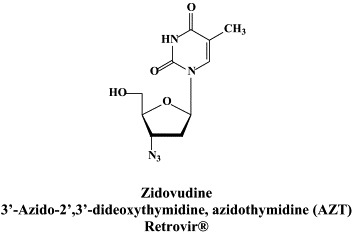
There are at present twenty some compounds available for the treatment of HIV infections.45 These compounds fall into 5 categories: (i) nucleoside reverse transcriptase inhibitors (NRTIs): zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir and emtricitabine; (ii) nucleotide reverse transcriptase inhibitors (NtRTIs): tenofovir disoproxil fumarate; (iii) non-nucleoside reverse transcriptase inhibitors (NNRTIs): nevirapine, delavirdine and efavirenz; (iv) protease inhibitors (PIs): saquinavir, ritonavir, indinavir, nelfinavir, amprenavir, lopinavir (combined at a 4 to 1 ratio with ritonavir), atazanavir, fosamprenavir, tipranavir and darunavir; and (v) fusion inhibitors (FIs): enfuvirtide. Several of these compounds are also available as fixed dose combinations: zidovudine with lamivudine (Combivir®), lamivudine with abacavir (Kivexa®), and emtricitabine with tenofovir disoproxil fumarate (Truvada®). A triple-drug fixed dose combination, containing efavirenz, emtricitabine and tenofovir disoproxil fumarate (Atripla®) has recently been launched.
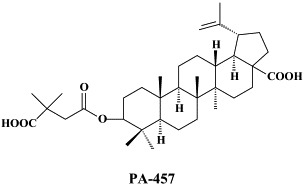
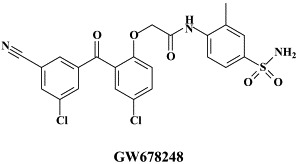
In addition to the 22 licensed anti-HIV compounds, various others are (or have been) in clinical [phase II (or III)] development: the HIV-1 attachment inhibitors BMS-378806 and BMS-488043,104 the CXCR4 antagonist AMD-3100 (as stem cell mobilizer for stem cell transplantation in patients with non-Hodgkin lymphoma or multiple myeloma),43 the CCR5 antagonists154 SCH-C, vicriviroc (SCH-D, SCH 417690),146 aplaviroc (873140)153 and maraviroc (UK-427,857),56 the NRTIs racivir, (−)-dOTC [AVX-754 (SPD-754), which has been accredited with activity against most other NRTI-resistant HIV-1 strains],69 reverset, elvucitabine, alovudine and amdoxovir, the NNRTIs capravirine and etravirine, the protease inhibitor (PI) darunavir (TMC-114)45., 52. (which, in the mean time, has been approved for clinical use), and the gag (p24) maturation inhibitor 3-O-(3′,3′-dimethylsuccinyl)-betulinic acid (PA-457).100 Also, a prodrug of the benzophenone GW 678248 has recently progressed to phase II clinical studies.133
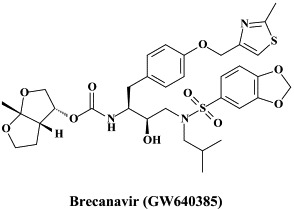
The protease inhibitor brecanavir (GW 640385) has progressed to phase II/III clinical trials.144 Although brecanavir can engender, on its own, in vitro drug resistance development,160 co-administration of brecanavir (300 mg) with ritonavir (100 mg) significantly increased the plasma brecanavir levels, achieving drug concentration predicted to inhibit protease inhibitor-resistant HIV mutants.63 Pharmacokinetic boosting with sub-therapeutic doses of ritonavir has become a standard procedure to enhance systemic drug exposures for a variety of the HIV protease inhibitors.
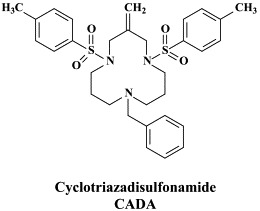
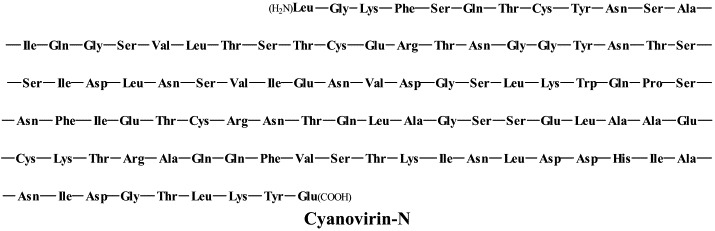
Yet other compounds are in preclinical development and/or may soon proceed to clinical phase I/II clinical studies: the CD4 (HIV receptor) down-modulator cyclotriazadisulfonamide (CADA);152 the HIV gp120 envelope-binding protein cyanovirin-N as a topical microbicide;18 KRH-2731, a CXCR4 antagonist, structurally related to KRH-1636;79 the CXCR4 antagonist AMD-070 (a derivative of the bicyclam AMD3100, which is currently being pursued in phase II/III clinical trials, in combination with granulocyte colony-stimulating factor (G-CSF), for the mobilization of autologous hematopoietic progenitor cells);62 TAK-220, a CCR5 antagonist, structurally related to TAK-779,5 which has proved to be a highly potent (orally bioavailable) inhibitor of CCR5-using (R5) HIV-1 strains,116., 147. and acts synergistically with other antiretrovirals;149 TAK-652, another orally bioavailable inhibitor of CCR5-mediated HIV infection.6
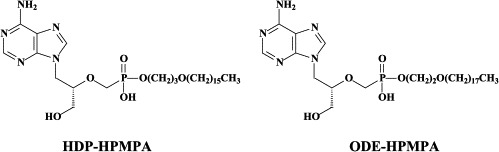
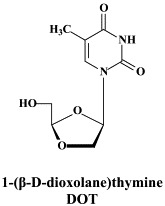
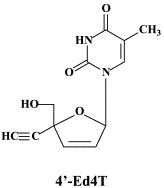
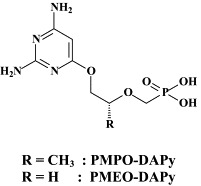
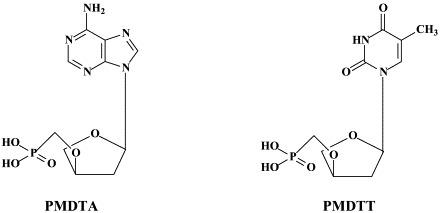
Noteworthy among the new NRTIs are MIV-210, a prodrug of the NRTI 3′-fluoro-2′,3′-dideoxyguanosine; the thymine dioxolane DOT, another NRTI;27 4′-Ed4T (2′,3′-didehydro-3′-deoxy-4′-ethynyl-2′-deoxythymidine), which has favorable oral bioavailabity and a unique drug resistance profile, different from that of the other NRTIs.117 Newly synthesized “phosphonate” analogues include the NtRTIs 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines PMPO-DAPy, PMEO-DAPy, and 5-substituted derivatives thereof,51 and the deoxythreosyl nucleoside phoshonates phosphonomethyldeoxythreosyladenine (PMDTA) and -thymine (PMDTT).156 Also alkoxyalkyl [i.e. hexadecyloxypropyl (HDP) and octadecyloxyethyl (ODE)] esters of the prototype “phosphonate”, (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]-adenine [HPMPA] have been reported to inhibit HIV-1 replication in vitro at nanomolar concentrations.77
Among the NNRTIs which have been further pursued for their anti-HIV potential, are thiocarboxanilide UC-781 and dapivirine (TMC-120), both as topical microbicides, and rilpivirine (R-278474), one of the most potent anti-HIV agents ever described;82 GW678248, a novel benzophenone NNRTI;61., 74. which has activity at 1 nM against the K103N and Y181C RT HIV-1 mutants associated with clinical resistance to efavirenz and nevirapine, respectively,133 the alkenyldiarylmethanes (ADAMS) with metabolically labile methylester moieties replaced by isoxazolone, isoxazole, oxazolone or cyano substituents.54
The polymerase activity of the HIV-1 reverse transcriptase (RT) is entirely dependent on the heterodimeric structure of the enzyme. RT dimerization, therefore, represents a molecular target for the development of new HIV inhibitors; it is the point of attack for the 2′,5′-bis-O-tert-butyldimethylsilyl-β-D-ribofuranosyl]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole-2″,2″-dioxide)-thymine (TSAO-T) derivatives, a class of compounds originally categorized under the NNRTIs.139
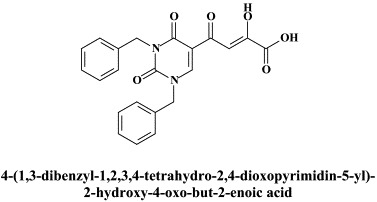
A number of compounds, among which the 1,6-naphthyridine-7-carboxamides L-870,810 and L-870,812, are targeted at the HIV-1 integrase.75., 76. Novel HIV-1 integrase inhibitors have been derived from quinolone antibiotic.136 Phase I/II clinical studies have been undertaken with GS-9137 (JTK-303), the prototype of this class of compounds.53 This compound showed an EC50 of 0.9 nM in an acute HIV-1 infection assay,136 and effected a reduction in viral load in short-term trials in short-term monotherapy trials.53 The 3,7-dihydroxytropolones represent an interesting platform for the design of inhibitors of both the reverse transcriptase (and RNase H) as well as the HIV integrase.55 Similarly, indolyl aryl sulfone may serve as a platform for the design of new NNRTIs effective against K103N HIV-1 variants.20 Recently, diketo acids bearing a nucleobase scaffold have been described as highly potent HIV integrase inhibitors:114 the prototype compound, 4-(1,3-dibenzyl-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-5-yl)-2-hydroxy-4-oxo-but-2-enoic acid, exhibited an anti-HIV selectivity index in cell culture of >4000. Meanwhile, an HIV vector-based single-cycle assay has been developed which should facilitate the evaluation of potential HIV integrase inhibitors.17
Among the PIs, novel HIV-1 protease inhibitors have been described which were designed specifically to interact with the backbone of HIV protease active site to combat drug resistance,66 and, among the triterpene (betulinic acid) derivatives new potent anti-HIV agents were reported164 to demonstrate a better antiviral profile than the prototype compound PA-457.100
In addition to the aforementioned cyanovirin-N, thiocarboxanilide UC-781 and dapivirine, there are some other compounds that could be further developed as topical (i.e. vaginal) microbicides, namely the aglycons of the glycopeptide antibiotics vancomycin, teicoplanin and eremomycin which specifically interact with the gp120 glycoprotein.126 Also the plant lectins, i.e. Galanthus nivalis agglutinin (GNA) and Hippeastrum hybrid agglutinin (HHA) represent potential candidate anti-HIV microbicides: they show marked stability at relatively low pH and high temperatures for prolonged time periods, they directly interact with the viral envelope and prevent entry of HIV into its target cells.7 Upon prolonged exposure of HIV in cell culture to HHA or GNA, the virus acquires resistance mutations in the gp120 glycoprotein which are predominantly located at the N-glycosylation (asparagine) sites.8
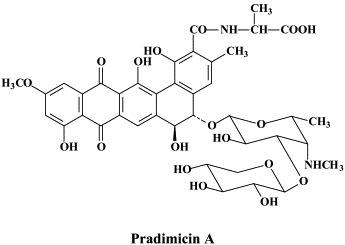
Cyanovirin-N and the plant lectins GNA and HHA can be termed carbohydrate-binding agents (CBAs); due to their carbohydrate-binding properties, they interact with the viral envelope glycoprotein, thereby preventing the HIV entry process. Recently, a non-peptidic benzonaphtacene quinone antibiotic, pradimicin A, has been described as a low-molecular-weight CBA that blocks HIV entry by specifically interacting with the mannose moieties of the HIV-1 gp120.9
An avenue to be further explored is the combination of different microbicides, such as the NNRTI thiocarboxanilide UC-781 with the cellulose acetate 1,2-benzenedicarboxylate (CAP) viral entry inhibitor, which exhibit synergistic and complementary effects against HIV-1 infection.102 There is, in addition, no shortage of sulfated and sulfonated polymers (starting off with suramin, the first polysulfonate ever shown to be active against HIV) which could be considered as topical anti-HIV microbicides.137
RNA interference (RNAi) may be considered a new powerful tool for intracellular immunization against HIV-1 infection. It has been demonstrated in short-term assays that HIV-1 replication can be inhibited by siRNAs directed against viral targets (i.e. rev) or cellular targets (i.e. CCR5).4., 97., 119., 127. As to the viral targets, siRNAs targeting conserved gag, pol, int, vpu regions21 or gp41, tat, rev or nef 33 were shown to inhibit virus production. Although targeting single HIV-1 sequences with siRNAs can result in strong inhibition of viral replication, it is likely followed by the escape of mutated viral variants.32 Therefore, antiviral approaches involving RNAi should be used in a combined fashion so as to prevent emergence of resistant viruses.
It is, furthermore, worth investigating whether RNAi can be harnessed for use in microbicides. As described above, an siRNA-based microbicide has been shown to protect mice from lethal HSV-2 infection.123 Extension of these results to the design of an HIV microbicide would also require demonstrating silencing in resident tissue macrophages, dendritic cells and T cells. Further considering the requirement of combining siRNAs that target multiple viral genes so as to cover viral sequence diversity and to prevent potential escape mutation, the development of an effective siRNA-based HIV microbicide may seem as a challenging task.
XII. Conclusion
About forty compounds are registered as antiviral drugs, at least half of which are used to treat HIV infections. An even greater number of compounds are under clinical or preclinical development, with again, as many targeting HIV as all the other viruses taken together. This implies that HIV, since its advent, has remained the main stay in antiviral drug development. Antiviral agents can, as guided by the anti-HIV agents as examples, be divided in roughly five categories:
-
(i)
nucleoside analogues,
-
(ii)
nucleotide analogues (or acyclic nucleoside phosphonates),
-
(iii)
non-nucleoside analogues,
-
(iv)
protease inhibitors, and
-
(v)
virus–cell fusion inhibitors.
Molecular targets are for (i) and (ii) the viral DNA polymerase (whether DNA-dependent as in the case of herpesviruses, or RNA-dependent as in the case of HIV or HBV); for (iii) RNA-dependent DNA polymerase (reverse transcriptase), associated with HIV, or RNA-dependent RNA polymerase (RNA replicase) associated with HCV; for (iv) the proteases associated with HIV and HCV; and for (v) the fusion process of HIV (and, potentially, other viruses such as the SARS coronavirus and RSV). Antiviral agents may also exert their antiviral effects through an interaction with cellular targets such as IMP dehydrogenase (ribavirin) and SAH hydrolase (3-deazaneplanocin A). The latter enzymes are essential for viral RNA synthesis (through the supply of GTP) and viral mRNA maturation (through 5′-capping), respectively. Finally, interferons (now generally provided in their pegylated form) may be advocated in the therapy of those viral infections (actually, HBV and HCV; prospectively, Coxsackie B, SARS, …) that, as yet, cannot be sufficiently curbed by other therapeutic measures.
Acknowledgements
I thank Mrs. Christiane Callebaut for her invaluable editorial assistance.
References
- 1.Andrei G., Snoeck R., Vandeputte M., De Clercq E. Activities of various compounds against murine and primate polyomaviruses. Antimicrob. Agents Chemother. 1997;41:587–593. doi: 10.1128/aac.41.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei G., Sienaert R., McGuigan C., De Clercq E., Balzarini J., Snoeck R. Susceptibilities of several clinical varicella-zoster virus (VZV) isolates and drug-resistant VZV strains to bicyclic furano pyrimidine nucleosides. Antimicrob. Agents Chemother. 2005;49:1081–1086. doi: 10.1128/AAC.49.3.1081-1086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrei G., Gammon D.B., Fiten P., De Clercq E., Opdenakker G., Snoeck R., Evans D.H. Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J. Virol. 2006;80:9391–9401. doi: 10.1128/JVI.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arteaga H.J., Hinkula J., van Dijk-Hard I., Dilber M.S., Wahren B., Christensson B., Mohamed A.J., Smith C.I. Choosing CCR5 or Rev siRNA in HIV-1. Nature Biotechnol. 2003;21:230–231. doi: 10.1038/nbt0303-230. [DOI] [PubMed] [Google Scholar]
- 5.Baba M., Nishimura O., Kanzaki N., Okamoto M., Sawada H., Iizawa Y., Siraishi M., Aramaki Y., Okonogi K., Ogawa Y., Meguro K., Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba M., Takashima K., Miyake H., Kanzaki N., Teshima K., Wang X., Shiraishi M., Iizawa Y. TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob. Agents Chemother. 2005;49:4584–4591. doi: 10.1128/AAC.49.11.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzarini J., Hatse S., Vermeire K., Princen K., Aquaro S., Perno C.F., De Clercq E., Egberink H., Vanden Mooter G., Peumans W., Van Damme E., Schols D. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzarini J., Van Laethem K., Hatse S., Vermeire K., De Clercq E., Peumans W., Van Damme E., Vandamme A.M., Bolmstedt A., Schols D. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J. Virol. 2004;78:10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balzarini J., Van Laethem K., Daelemans D., Hatse S., Bugatti A., Rusnati M., Igarashi Y., Oki T., Schols D. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J. Virol. 2007;81:362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beadle J.R., Hartline C., Aldern K.A., Rodriguez N., Harden E., Kern E.R., Hostetler K.Y. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 2002;46:2381–2386. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beadle J.R., Wan W.B., Ciesla S.L., Keith K.A., Hartline C., Kern E.R., Hostetler K.Y. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine against cytomegalovirus and orthopoxviruses. J. Med. Chem. 2006;49:2010–2015. doi: 10.1021/jm050473m. [DOI] [PubMed] [Google Scholar]
- 12.Benhamou Y., Fleury H., Trimoulet P., Pellegrin I., Urbinelli R., Katlama C., Rozenbaum W., Le Teuff G., Trylesinski A., Piketty C. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 13.Betz U.A., Fischer R., Kleymann G., Hendrix M., Rübsamen-Waigmann H. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57-1293. Antimicrob. Agents Chemother. 2002;46:1766–1772. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biron K.K. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71:154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Biron K.K., Harvey R.J., Chamberlain S.C., Good S.S., Smith A.A., 3rd, Davis M.G., Talarico C.L., Miller W.H., Ferris R., Dornsife R.E., Stanat S.C., Drach J.C., Townsend L.B., Koszalka G.W. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 2002;46:2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S. Biswas, L. Jennens, H.J. Field, The helicase primase inhibitor, BAY 57-1293 shows potent therapeutic antiviral activity superior to famciclovir in BALB/c mice infected with herpes simplex virus type 1, Antiviral Res., in press [DOI] [PubMed]
- 17.Bona R., Andreotti M., Buffa V., Leone P., Galluzzo C.M., Amici R., Palmisano L., Mancini M.G., Michelini Z., Di Santo R., Costi R., Roux A., Pommier Y., Marchand C., Vella S., Cara A. Development of a human immunodeficiency virus vector-based single-cycle assay for evaluation of anti-integrase compounds. Antimicrob. Agents Chemother. 2006;50:3407–3417. doi: 10.1128/AAC.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd M.R., Gustafson K.R., McMahon J.B., Shoemaker R.H., O'Keefe B.R., Mori T., Gulakowski R.J., Wu L., Rivera M.I., Laurencot C.M., Currens M.J., Cardellina J.H., 2nd, Buckheit R.W., Jr, Nara P.L., Pannell L.K., Sowder R.C., 2nd, Henderson L.E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulller R.M., Owens G., Schriewer J., Melman L., Beadle J.R., Hostetler K.Y. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–481. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Cancio R., Silvestri R., Ragno R., Artico M., De Martino G., La Regina G., Crespan E., Zanoli S., Hubscher U., Spadari S., Maga G. High potency of indolyl aryl sulfone nonnucleoside inhibitors towards drug-resistant human immunodeficiency virus type 1 reverse transcriptase mutants is due to selective targeting of different mechanistic forms of the enzyme. Antimicrob. Agents Chemother. 2005;49:4546–4554. doi: 10.1128/AAC.49.11.4546-4554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L.-J., Liu X., He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 2005;12:1133–1144. doi: 10.1038/sj.gt.3302509. [DOI] [PubMed] [Google Scholar]
- 22.Chang T.-T., Gish R.G., de Man R., Gadano A., Sollano J., Chao Y.C., Lok A.S., Han K.H., Goodman Z., Zhu J., Cross A., DeHertogh D., Wilber R., Colonno R., Apelian D. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y.-C., Ying C.-X., Leung C.-H., Li Y. New targets and inhibitors of HBV replication to combat drug resistance. J. Clin. Virol. 2005;34(Suppl. 1):S147–S150. doi: 10.1016/s1386-6532(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 24.Cho J.H., Bernard D.L., Sidwell R.W., Kern E.R., Chu C.K. Synthesis of cyclopentenyl carbocyclic nucleosides as potential antiviral agents against orthopoxviruses and SARS. J. Med. Chem. 2006;49:1140–1148. doi: 10.1021/jm0509750. [DOI] [PubMed] [Google Scholar]
- 25.Chou S., Marousek G.I. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob. Agents Chemother. 2006;50:3470–3472. doi: 10.1128/AAC.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou S., Van Wechel L.C., Marousek G.I. Effect of cell culture conditions on the anticytomegalovirus activity of maribavir. Antimicrob. Agents Chemother. 2006;50:2557–2559. doi: 10.1128/AAC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu C.K., Yadav V., Chong Y.H., Schinazi R.F. Anti-HIV activity of (−)-(2R,4R)-1-(2-hydroxymethyl-1,3-dioxolan-4-yl)-thymine against drug-resistant HIV-1 mutants and studies of its molecular mechanism. J. Med. Chem. 2005;48:3949–3952. doi: 10.1021/jm050060l. [DOI] [PubMed] [Google Scholar]
- 28.Ciesla S.L., Trahan J., Wan W.B., Beadle J.R., Aldern K.A., Painter G.R., Hostetler K.Y. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 29.Crute J.J., Tsurumi T., Zhu L., Weller S.K., Olivo P.D., Challberg M.D., Mocarski E.S., Lehman I.R. Herpes simplex virus 1 helicase–primase: a complex of three herpes-encoded gene products. Proc. Natl. Acad. Sci. USA. 1989;86:2186–2189. doi: 10.1073/pnas.86.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crute J.J., Grygon C.A., Hargrave K.D., Simoneau B., Faucher A.M., Bolger G., Kibler P., Liuzzi M., Cordingley M.G. Herpes simplex virus helicase–primase inhibitors are active in animal models of human disease. Nature Med. 2002;8:386–391. doi: 10.1038/nm0402-386. [DOI] [PubMed] [Google Scholar]
- 31.Dal Pozzo F., Andrei G., Holý A., Van Den Oord J., Scagliarini A., De Clercq E., Snoeck R. Activities of acyclic nucleoside phosphonates against Orf virus in human and ovine cell monolayers and organotypic ovine raft cultures. Antimicrob. Agents Chemother. 2005;49:4843–4852. doi: 10.1128/AAC.49.12.4843-4852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das A.T., Brummelkamp T.R., Westerhout E.M., Vink M., Madiredjo M., Bernards R., Berkhout B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J. Virol. 2004;78:2601–2605. doi: 10.1128/JVI.78.5.2601-2605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave R.S., Pomerantz R.J. Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains. J. Virol. 2004;78:13687–13696. doi: 10.1128/JVI.78.24.13687-13696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dave R.S., McGettigan J.P., Qureshi T., Schnell M.J., Nunnari G., Pomerantz R.J. siRNA targeting vaccinia virus double-stranded RNA binding protein [E3L] exerts potent antiviral effects. Virology. 2006;348:489–497. doi: 10.1016/j.virol.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 35.De Bolle L., Andrei G., Snoeck R., Zhang Y., Van Lommel A., Otto M., Bousseau A., Roy C., De Clercq E., Naesens L. Potent, selective and cell-mediated inhibition of human herpesvirus 6 at an early stage of viral replication by the non-nucleoside compound CMV423. Biochem. Pharmacol. 2004;67:325–336. doi: 10.1016/j.bcp.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 36.De Bolle L., Naesens L., De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Clercq E. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 2001;14:382–397. doi: 10.1128/CMR.14.2.382-397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Clercq E. Clinical potential of acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin. Microbiol. Rev. 2003;16:569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Clercq E. Highly potent and selective inhibition of varicella-zoster virus replication by bicyclic furo [2,3-d]pyrimidine nucleoside analogues. Med. Res. Rev. 2003;23:253–274. doi: 10.1002/med.10035. [DOI] [PubMed] [Google Scholar]
- 41.De Clercq E. New inhibitors of HCMV (human cytomegalovirus) on the horizon. J. Antimicrob. Chemother. 2003;51:1079–1083. doi: 10.1093/jac/dkg205. [DOI] [PubMed] [Google Scholar]
- 42.De Clercq E. Potential of acyclic nucleoside phosphonates in the treatment of DNA virus and retrovirus infections. Expert Rev. Anti-infect. Ther. 2003;1:21–43. doi: 10.1586/14787210.1.1.21. [DOI] [PubMed] [Google Scholar]
- 43.De Clercq E. The bicyclam AMD3100 story. Nature Rev. Drug Discovery. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 44.De Clercq E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 45.De Clercq E. Emerging anti-HIV drugs. Expert Opin. Emerging Drugs. 2005;10:241–274. doi: 10.1517/14728214.10.2.241. [DOI] [PubMed] [Google Scholar]
- 46.De Clercq E., Field H.J. Antiviral prodrugs—the development of successful prodrug strategies for antiviral chemotherapy. Brit. J. Pharmacol. 2005;147:1–11. doi: 10.1038/sj.bjp.0706446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Clercq E., Naesens L. In search of effective anti-HHV-6 agents. J. Clin. Virol. 2006;(Suppl.):S82–S86. doi: 10.1016/S1386-6532(06)70017-8. [DOI] [PubMed] [Google Scholar]
- 48.De Clercq E., Neyts J. Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev. Med. Virol. 2004;14:289–300. doi: 10.1002/rmv.439. [DOI] [PubMed] [Google Scholar]
- 49.De Clercq E., Andrei G., Snoeck R., De Bolle L., Naesens L., Degrève B., Balzarini J., Zhang Y., Schols D., Leyssen P., Ying C., Neyts J. Acyclic/carbocyclic guanosine analogues as anti-herpesvirus agents. Nucleosides, Nucleotides & Nucleic Acids. 2001;20:271–285. doi: 10.1081/NCN-100002298. [DOI] [PubMed] [Google Scholar]
- 50.De Clercq E., Naesens L., De Bolle L., Schols D., Zhang Y., Neyts J. Antiviral agents active against human herpesviruses HHV-6, HHV-7, HHV-7 and HHV-8. Rev. Med. Virol. 2001;11:381–395. doi: 10.1002/rmv.336. [DOI] [PubMed] [Google Scholar]
- 51.De Clercq E., Andrei G., Balzarini J., Leyssen P., Naesens L., Neyts J., Pannecouque C., Snoeck R., Ying C., Hocková D., Holý A. Antiviral potential of a new generation of acyclic nucleoside phosphonates, the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines. Nucleosides, Nucleotides & Nucleic Acids. 2005;24:331–341. doi: 10.1081/ncn-200059772. [DOI] [PubMed] [Google Scholar]
- 52.De Meyer S., Azijn H., Surleraux D., Jochmans D., Tahri A., Pauwels R., Wigerinck M.-P., de Béthune P. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 2005;49:2314–2321. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeJesus E., Berger D., Markowitz M., Cohen C., Hawkins T., Ruane P., Elion R., Farthing C., Zhong L., Cheng A.K., McColl D., Kearney B.P. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-native and treatment-experienced patients. J. Acquir. Immune Defic. Syndr. 2006;43:1–5. doi: 10.1097/01.qai.0000233308.82860.2f. [DOI] [PubMed] [Google Scholar]
- 54.Deng B.-L., Hartman T.L., Buckheit R.W., Jr, Pannecouque C., De Clercq E., Cushman M. Replacement of the metabolically labile methyl esters in the alkenyldiarylmethane series of non-nucleoside reverse transcriptase inhibitors with isoxazolone, isoxazole, oxazolone, or cyano substituents. J. Med. Chem. 2006;49:5316–5323. doi: 10.1021/jm060449o. [DOI] [PubMed] [Google Scholar]
- 55.Didierjean J., Isel C., Querré F., Mouscadet J.F., Aubertin A.M., Valnot J.Y., Piettre S.R., Marquet R. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, RNase H, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother. 2005;49:4884–4894. doi: 10.1128/AAC.49.12.4884-4894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorr P., Westby M., Dobbs S. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan J., Liuzzi M., Paris W., Liard F., Browne A., Dansereau N., Simoneau B., Faucher A.-M., Cordingley M.G. Oral bioavailability and in vivo efficacy of the helicase–primase inhibitor BILS 45 BS against acyclovir-resistant herpes simplex virus type 1. Antimicrob. Agents Chemother. 2003;47:1798–1804. doi: 10.1128/AAC.47.6.1798-1804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan X., Zhang X., Zhou L., Keith K.A., Kern E.R., Torrence P.F. 5-(Dimethoxymethyl)-2′-deoxyuridine: a novel gem diether nucleoside with anti-orthopoxvirus activity. J. Med. Chem. 2006;49:3377–3382. doi: 10.1021/jm0601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan X., Zhang X., Zhou L., Keith K.A., Kern E.R., Torrence P.F. A pyrimidine-pyrazolone nucleoside chimera with potent in vitro anti-orthopoxvirus activity. Bioorg. Med. Chem. Lett. 2006;16:3224–3228. doi: 10.1016/j.bmcl.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 60.Faucher A.M., White P.W., Brochu C., Grand-Maitre C., Rancourt J., Fazal G. Discovery of small-molecule inhibitors of the ATPase activity of human papillomavirus E1 helicase. J. Med. Chem. 2004;47:18–21. doi: 10.1021/jm034206x. [DOI] [PubMed] [Google Scholar]
- 61.Ferris R.G., Hazen R.J., Roberts G.B., St. Clair M.H., Chan J.H., Romines K.R., Freeman G.A., Tidwell J.H., Schaller L.T., Cowan J.R., Short S.A., Weaver K.L., Selleseth D.W., Moniri K.R., Boone L.R. Antiviral activity of GW678248, a novel benzophenone nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 2005;49:4046–4051. doi: 10.1128/AAC.49.10.4046-4051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flomenberg N., Devine S.M., DiPersio J.F., Liesveld J.L., McCarty J.M., Rowley S.D., Vesole K., Badel D.H., Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 63.Ford S.L., Reddy Y.S., Anderson M.T., Murray S.C., Fernandez P., Stein D.S., Johnson M.A. Single-dose safety and pharmacokinetics of brecanavir, a novel human immunodeficiency virus protease inhibitor. Antimicrob. Agents Chemother. 2006;50:2201–2206. doi: 10.1128/AAC.01490-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fung S.K., Andreone P., Han S.H., Reddy K.R., Regev A., Keeffe E.B., Hussain M., Cursaro C., Richtmyer P., Marrero J.A., Lok A.S.F. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J. Hepatol. 2005;43:937–943. doi: 10.1016/j.jhep.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 65.Fung S.K., Chae H.B., Fontana R.J., Conjeevaram H., Marrero J., Oberhelman K., Hussain M., Lok A.S.F. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J. Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh A.K., Sridhar P.R., Leshchenko S., Hussain A.K., Li J., Kovalevsky A.Y., Walters D.E., Widekind J.E., Grum-Tokars V., Das D., Koh Y., Maeda K., Gatanaga H., Weber I.T., Mitsuya H. Structure-based design of novel HIV-1 protease inhibitors to combat drug resistance. J. Med. Chem. 2006;49:5252–5261. doi: 10.1021/jm060561m. [DOI] [PubMed] [Google Scholar]
- 67.Giladi H., Ketzinel-Gilad M., Rivkin L., Felig Y., Nussbaum O., Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 2003;8:769–776. doi: 10.1016/s1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 68.Golankiewicz B., Ostrowski T. Tricyclic nucleoside analogues as antiherpes agents. Antiviral Res. 2006;71:134–140. doi: 10.1016/j.antiviral.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Gu Z., Allard B., de Muys J.M., Lippens J., Rando R.F., Nguyen-Ba N., Ren C., McKenna P., Taylor D.L., Bethell R.C. In vitro antiretroviral activity and in vitro toxicity profile of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor for treatment of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2006;50:625–631. doi: 10.1128/AAC.50.2.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.T., Kitis G., Rizzetto M., Marcellin P., Lim S.G., Goodman Z., Wulfsohn M.S., Xiong S., Fry J., Brosgart C.L. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 71.Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.T., Kitis G., Rizzetto M., Marcellin P., Lim S.G., Goodman Z., Ma J., Arterburn S., Xiong S., Currie G., Brosgart C.L. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 72.Han Y.X., Xue R., Zhao W., Zhou Z.X., Li H.S., Chen J.N., Chen Y.L., Wang X.H., Li Y.H., Wu Y.W., You X.F., Zhao L.X., Jiang J.D. Antiviral therapeutic efficacy of foscarnet in hepatitis B virus infection. Antiviral Res. 2005;68:147–153. doi: 10.1016/j.antiviral.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Hartline C.B., Gustin K.M., Wan W.B., Ciesla S.L., Beadle J.R., Hostetler K.Y., Kern E.R. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 2005;191:396–399. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
- 74.Hazen R.J., Harvey R.J., St. Clair M.H., Ferris R.G., Freeman G.A., Tidwell J.H., Schaller L.T., Cowan J.R., Short S.A., Romines K.R., Chan J.H., Boone L.R. Anti-human immunodeficiency virus type 1 activity of the nonnucleoside reverse transcriptase inhibitor GW678248 in combination with other antiretrovirals against clinical isolate viruses and in vitro selection for resistance. Antimicrob. Agents Chemother. 2005;49:4465–4473. doi: 10.1128/AAC.49.11.4465-4473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hazuda D.J., Anthony N.J., Gomez R.P., Jolly S.M., Wai J.S., Zhuang L., Fisher T.E., Embrey M., Guare J.P., Jr, Egbertson M.S., Vacca J.P., Huff J.R., Felock P.J., Witmer M.V., Stillmock K.A., Danovich R., Grobler J., Miller M.D., Espeseth A.S., Jin L., Chen I.W., Lin J.H., Kassahun K., Ellis J.D., Wong B.K., Xu W., Pearson P.G., Schleif W.A., Cortese R., Emini E., Summa V., Holloway M.K., Young S.D. A naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integrase. Proc. Natl. Acad. Sci. USA. 2004;101:11233–11238. doi: 10.1073/pnas.0402357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hazuda D.J., Young S.D., Guare J.P., Anthony N.J., Gomez R.P., Wai J.S., Vacca J.P., Handt L., Motzel S.L., Klein H.J., Dornadula G., Danovich R.M., Witmer M.V., Wilson K.A., Tussey L., Schleif W.A., Gabryelski L.S., Jin L., Miller M.D., Casimiro D.R., Emini E.A., Shiver J.W. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- 77.Hostetler K.Y., Aldern K.A., Wan W.B., Ciesla S.L., Beadle J.R. Alkoxyalkyl esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine are potent inhibitors of the replication of wild-type and drug-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 2006;50:2857–2859. doi: 10.1128/AAC.01223-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu R., Li L., Degrève B., Dutschman G.E., Lam W., Cheng Y.-C. Behavior of thymidylate kinase toward monophosphate metabolites and its role in the metabolism of 1-(2′-deoxy-2′-fluoro-beta-L-arabinofuranosyl)-5-methyluracil (Clevudine) and 2',3′-didehydro-2',3′-dideoxythymidine in cells. Antimicrob. Agents Chemother. 2005;49:2044–2049. doi: 10.1128/AAC.49.5.2044-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ichiyama K., Yokoyama-Kumakura S., Tanaka Y., Tanaka R., Hirose K., Bannai K., Edamatsu T., Yanaka M., Niitani Y., Miyano-Kurosaki N., Takaku H., Koyanagi Y., Yamamoto N. A duodenally absorbable CXC chemokine receptor 4 antagonist, KRH-1636, exhibits a potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA. 2003;100:4185–4190. doi: 10.1073/pnas.0630420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacquard A.-C., Brunelle M.-N., Pichoud C., Durantel D., Carrouee-Durantel S., Trépo C., Zoulim F. In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2',3′-dideoxy-3′-fluoroguanosine. Antimicrob. Agents Chemother. 2006;50:955–961. doi: 10.1128/AAC.50.3.955-961.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janssen H.L.A., van Zonneveld M., Senturk H., Zeuzem S., Akarca U.S., Cakaloglu Y., Simon C., So T.M., Gerken G., de Man R.A., Niesters H.G., Zondervan P., Hansen B., Schalm S.W. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 82.Janssen P.A., Lewi P.J., Arnold E., Daeyaert F., de Jonge M., Heeres J., Koymans L., Vinkers M., Guillemont J., Pasquier E., Kukla M., Ludovici D., Andries K., de Béthune M.-P., Pauwels R., Das K., Clark A.D., Jr, Frenkel Y.V., Hughes S.H., Medaer B., De Knaep F., Bohets H., De Clerck F., Lampo A., Williams S., Stoffels P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J. Med. Chem. 2005;48:1901–1909. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 83.Kern E.R., Hartline C., Harden E., Keith K., Rodriguez N., Beadle J.R., Hostetler K.Y. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kern E.R., Bidanset D.J., Hartline C.B., Yan Z., Zemlicka J., Quenelle D.C. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob. Agents Chemother. 2004;48:4745–4753. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kern E.R., Kushner N.L., Hartline C.B., Williams-Aziz S.L., Harden E.A., Zhou S., Zemlicka J., Prichard M.N. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob. Agents Chemother. 2005;49:1039–1045. doi: 10.1128/AAC.49.3.1039-1045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein C., Bock C.T., Wedemeyer H., Wüstefeld T., Locarnini S., Dienes H.P., Kubicka S., Manns M.P., Trautwein C. Inhibition of hepatitis B virus replication in vivo by nucleoside analogues and siRNA. Gastroenterology. 2003;125:9–18. doi: 10.1016/s0016-5085(03)00720-0. [DOI] [PubMed] [Google Scholar]
- 87.Kleymann G., Fischer R., Betz U.A., Hendrix M., Bender W., Schneider G., Handke U., Eckenberg P., Hewlett G., Pevzner V., Baumeister J., Weber O., Henninger K., Keldenich J., Jensen A., Kolb J., Bach U., Popp A., Maben J., Frappa I., Haebich D., Lockhoff O., Rübsamen-Waigmann H. New helicase–primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nature Med. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 88.Kornbluth R.S., Smee D.F., Sidwell R.W., Snarsky V., Evans D.H., Hostetler K.Y. Mutations in the E9L polymerase gene of cidofovir-resistant vaccinia virus strain WR are associated with the drug resistance phenotype. Antimicrob. Agents Chemother. 2006;50:4038–4043. doi: 10.1128/AAC.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koszalka G.W., Johnson N.W., Good S.S., Boyd L., Chamberlain S.C., Townsend L.B., Drach J.C., Biron K.K. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2002;46:2373–2380. doi: 10.1128/AAC.46.8.2373-2380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krosky P.M., Baek M.C., Coen D.M. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 2003;77:905–914. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai C.L., Rosmawati M., Lao J., Van Vlierberghe F.H., Anderson H., Thomas N., Dehertogh D. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002;123:1831–1838. doi: 10.1053/gast.2002.37058. [DOI] [PubMed] [Google Scholar]
- 92.Lai C.-L., Shouval D., Lok A.S., Chang T.T., Cheinquer H., Goodman Z., DeHertogh D., Wilber R., Zink R.C., Cross A., Colonno R., Fernandes L. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 93.Lalezari J.P., Aberg J.A., Wang L.H., Wire M.B., Miner R., Snowden W., Talarico C.L., Shaw S., Jacobson M.A., Drew W.L. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob. Agents Chemother. 2002;46:2969–2976. doi: 10.1128/AAC.46.9.2969-2976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lampertico P., Viganò M., Manenti E., Iavarone M., Lunghi G., Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414–1419. doi: 10.1002/hep.20939. [DOI] [PubMed] [Google Scholar]
- 95.Lebeau I., Andrei G., Dal Pozzo F., Beadle J.R., Hostetler K.Y., De Clercq E., van den Oord J., Snoeck R. Activities of alkoxyalkyl esters of cidofovir (CDV), cyclic CDV, and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine against orthopoxviruses in cell monolayers and in organotypic cultures. Antimicrob. Agents Chemother. 2006;50:2525–2529. doi: 10.1128/AAC.01489-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee H.-S., Chung Y.-H., Lee K., Byun K.S., Paik S.W., Han J.-Y., Yoo K., Yoo H.-W., Lee J.H., Yoo B.C. A 12-week clevudine therapy showed potent and durable antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2006;43:982–988. doi: 10.1002/hep.21166. [DOI] [PubMed] [Google Scholar]
- 97.Lee N.S., Dohjima T., Bauer G., Li M.J., Li H., Ehsani A., Salvaterra P., Rossi J. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nature Biotechnol. 2002;20:500–505. doi: 10.1038/nbt0502-500. [DOI] [PubMed] [Google Scholar]
- 98.Lee Y.-S., Suh D.J., Lim Y.-S., Jung S.W., Kim K.M., Lee H.C., Chung Y.-H., Lee Y.S., Yoo W., Kim S.-O. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 99.Levine S., Hernandez D., Yamanaka G., Zhang S., Rose R., Weinheimer S., Colonno R.J. Efficacies of entecavir against lamivudine-resistant hepatitis B virus replication and recombinant polymerases in vitro. Antimicrob. Agents Chemother. 2002;46:2525–2532. doi: 10.1128/AAC.46.8.2525-2532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li F., Goila-Gaur R., Salzwedel K., Kilgore N.R., Reddick M., Matallana C., Castillo A., Zoumplis D., Martin D.E., Orenstein J.M., Allaway G.P., Freed E.O., Wild C.T. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc. Natl. Acad. Sci. USA. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C.-J., Lai M.-Y., Chao Y.-C., Liao L.-Y., Yang S.-S., Hsiao T.-J., Hsieh T.-Y., Lin C.-L., Hu J.-T., Chen C.-L., Chen P.-J., Kao J.-H., Chen D.-S. Interferon α-2b with and without ribavirin in the treatment of hepatitis B e antigen-positive chronic hepatitis B: a randomized study. Hepatology. 2006;43:742–749. doi: 10.1002/hep.21100. [DOI] [PubMed] [Google Scholar]
- 102.Liu S., Lu H., Neurath A.R., Jiang S. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 2005;49:1830–1836. doi: 10.1128/AAC.49.5.1830-1836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liuzzi M., Kibler P., Bousquet C., Harji F., Bolger G., Garneau M., Lapeyre N., McCollum R.S., Faucher A.-M., Simoneau B., Cordingley M.G. Isolation and characterization of herpes simplex virus type 1 resistant to aminothiazolylphenyl-based inhibitors of the viral helicase–primase. Antiviral Res. 2004;64:161–170. doi: 10.1016/j.antiviral.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Madani N., Perdigoto A.L., Srinivasan K. Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J. Virol. 2004;78:3742–3752. doi: 10.1128/JVI.78.7.3742-3752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magee W.C., Hostetler K.Y., Evans D.H. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob. Agents Chemother. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marcellin P., Asselah T. Resistance to adefovir A: a new challenge in the treatment of chronic hepatitis B. J. Hepatol. 2005;43:920–923. doi: 10.1016/j.jhep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 107.Marcellin P., Chang T.T., Lim S.G., Tong M.J., Sievert W., Shiffman M.L., Jeffers L., Goodman Z., Wulfsohn M.S., Xiong S., Fry J., Brosgart C.L. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 108.Matthews S.J. Entecavir for the treatment of chronic hepatitis B virus infection. Clin. Ther. 2006;28:184–203. doi: 10.1016/j.clinthera.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 109.McCaffrey A.P., Nakai H., Pandey K., Huang Z., Salazar F.H., Xu H., Wieland S.F., Marion P.I., Kay M.A. Inhibition of hepatitis B virus in mice by RNA interference. Nature Biotechnol. 2003;21:639–644. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- 110.McGuigan C., Barucki H., Blewett S., Carangio A., Erichsen J.T., Andrei G., Snoeck R., De Clercq E., Balzarini J. Highly potent and selective inhibition of varicella-zoster virus by bicyclic furo pyrimidine nucleosides bearing an aryl side chain. J. Med. Chem. 2000;43:4993–4997. doi: 10.1021/jm000210m. [DOI] [PubMed] [Google Scholar]
- 111.Mentel R., Wegner U. Evaluation of the efficacy of -dideoxycytidine against adenovirus infection in a mouse pneumonia model. Antiviral Res. 2000;47:79–87. doi: 10.1016/s0166-3542(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 112.Naesens L., Bonnafous P., Agut H., De Clercq E. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 2006;(Suppl.):S69–S75. doi: 10.1016/S1386-6532(06)70015-4. [DOI] [PubMed] [Google Scholar]
- 113.Naesens L., Lenaerts L., Andrei G., Snoeck R., Van Beers D., Holý A., Balzarini J., De Clercq E. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 2005;49:1010–1016. doi: 10.1128/AAC.49.3.1010-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nair V., Chi G., Ptak R., Neamati N. HIV integrase inhibitors with nucleobase scaffolds: discovery of a highly potent anti-HIV agent. J. Med. Chem. 2006;49:445–447. doi: 10.1021/jm0508890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neyts J., Leyssen P., Verbeken E., De Clercq E. Efficacy of cidofovir in a murine model for disseminated/progressive vaccinia. Antimicrob. Agents Chemother. 2004;48:2267–2273. doi: 10.1128/AAC.48.6.2267-2273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishikawa M., Takashima K., Nishi T., Furuta R.A., Kanzaki N., Yamamoto Y., Fujisawa J. Analysis of binding sites for the new small-molecule CCR5 antagonist TAK-220 on human CCR5. Antimicrob. Agents Chemother. 2005;49:4708–4715. doi: 10.1128/AAC.49.11.4708-4715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nitanda T., Wang X., Kumamoto H., Haraguchi K., Tanaka H., Cheng Y.C., Baba M. Anti-human immunodeficiency virus type 1 activity and resistance profile of 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine in vitro. Antimicrob. Agents Chemother. 2005;49:3355–3360. doi: 10.1128/AAC.49.8.3355-3360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niu X.Y., Peng Z.L., Duan W.Q., Wang P., Wang H. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int. J. Gynecol. Cancer. 2006;16:743–751. doi: 10.1111/j.1525-1438.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 119.Novina C.D., Murray M.F., Dykxhoorn D.M., Beresford P.J., Riess J., Lee S.K., Collman R.G., Lieberman J., Shankar P., Sharp P.A. siRNA-directed inhibition of HIV-1 infection. Nature Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- 120.Osborn M.K., Lok A.S.F. Antiviral options for the treatment of chronic hepatitis B. J. Antimicrob. Chemother. 2006;57:1030–1034. doi: 10.1093/jac/dkl123. [DOI] [PubMed] [Google Scholar]
- 121.Osiowy C., Villeneuve J.-P., Heathcote E.J., Giles E., Borlang J. Detection of rtN236T and rtA181V/T mutations associated with resistance to adefovir dipivoxil in samples from patients with chronic hepatitis B virus infection by the INNO-LiPA HBV DR Line Probe Assay (version 2) J. Clin. Microbiol. 2006;44:1994–1997. doi: 10.1128/JCM.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Painter G.R., Hostetler K.Y. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 2004;22:423–427. doi: 10.1016/j.tibtech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 123.Palliser D., Chowdhury D., Wang Q.-Y., Lee S.J., Bronson R.T., Knipe D.M., Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 124.Peng J., Zhao Y., Mai J., Pang W.K., Wei X., Zhang P., Xu Y. Inhibition of hepatitis B virus replication by various RNAi constructs and their pharmacodynamic properties. J. Gen. Virol. 2005;86:3227–3234. doi: 10.1099/vir.0.81171-0. [DOI] [PubMed] [Google Scholar]
- 125.Prichard M.N., Keith K.A., Quenelle D.C., Kern E.R. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob. Agents Chemother. 2006;50:1336–1341. doi: 10.1128/AAC.50.4.1336-1341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Printsevskaya S.S., Solovieva S.E., Olsufyeva E.N., Mirchink E.P., Isakova E.B., De Clercq E., Balzarini J., Preobrazhenskaya M.N. Structure-activity relationship studies of a series of antiviral and antibacterial aglycon derivatives of the glycopeptide antibiotics vancomycin, eremomycin, and dechloroeremomycin. J. Med. Chem. 2005;48:3885–3890. doi: 10.1021/jm0500774. [DOI] [PubMed] [Google Scholar]
- 127.Qin X.F., An D.S., Chen I.S., Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quenelle D.C., Collins D.J., Wan W.B., Beadle J.R., Hostetler K.Y., Kern E.R. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quenelle D.C., Keith K.A., Kern E.R. In vitro and in vivo evaluation of isatin-β-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antiviral Res. 2006;71:24–30. doi: 10.1016/j.antiviral.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 130.Randhawa P., Farasati N.A., Shapiro R., Hostetler K.Y. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob. Agents Chemother. 2006;50:1564–1566. doi: 10.1128/AAC.50.4.1564-1566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reeves P.M., Bommarius B., Lebeis S., McNulty S., Christensen J., Swimm A., Chahroudi A., Chavan R., Feinberg M.B., Veach D., Bornmann W., Sherman M., Kalman D. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nature Med. 2005;11:731–739. doi: 10.1038/nm1265. [DOI] [PubMed] [Google Scholar]
- 132.Robbins S.J., Jackson R.J., Fenner F., Beaton S., Medveczky J., Ramshaw I.A., Ramsay A.J. The efficacy of cidofovir treatment of mice infected with ectromelia (mousepox) virus encoding interleukin-4. Antiviral Res. 2005;66:1–7. doi: 10.1016/j.antiviral.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Romines K.R., Freeman G.A., Schaller L.T., Cowan J.R., Gonzales S.S., Tidwell J.H., Andrews C.W., 3rd, Stammers D.K., Hazen R.J., Ferris R.G., Short S.A., Chan J.H., Boone L.R. Structure-activity relationship studies of novel benzophenones leading to the discovery of a potent, next generation HIV nonnucleoside reverse transcriptase inhibitor. J. Med. Chem. 2006;49:727–739. doi: 10.1021/jm050670l. [DOI] [PubMed] [Google Scholar]
- 134.Russ P., Schelling P., Scapozza L., Folkers G., De Clercq E., Marquez V.E. Synthesis and biological evaluation of 5-substituted derivatives of the potent antiherpes agent (North)-methanocarbathymine. J. Med. Chem. 2003;46:5045–5054. doi: 10.1021/jm030241s. [DOI] [PubMed] [Google Scholar]
- 135.Saag M.S. Emtricitabine, a new antiretroviral agent with activity against HIV and hepatitis B virus. Clin. Infect. Dis. 2006;42:126–131. doi: 10.1086/498348. [DOI] [PubMed] [Google Scholar]
- 136.Sato M., Motomura T., Aramaki H., Matsuda T., Yamashita M., Ito Y., Kawakami H., Matsuzaki Y., Watanabe W., Yamataka K., Ikeda S., Kodama E., Matsuoka M., Shinkai H. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J. Med. Chem. 2006;49:1506–1508. doi: 10.1021/jm0600139. [DOI] [PubMed] [Google Scholar]
- 137.Scordi-Bello I.A., Mosoian A., He C., Chen Y., Cheng Y., Jarvis G.A., Keller M.J., Hogarty K., Waller D.P., Profy A.T., Herold B.C., Klotman M.E. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob. Agents Chemother. 2005;49:3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shankar P., Manjunath N., Lieberman J. The prospect of silencing disease using RNA interference. JAMA. 2005;293:1367–1373. doi: 10.1001/jama.293.11.1367. [DOI] [PubMed] [Google Scholar]
- 139.Sluis-Cremer N., Hamamouch N., San Félix A., Velázquez S., Balzarini J., Camarasa M.-J. Structure-activity relationships of [-bis-O-(tert-butyldimethylsilyl)-beta-D-ribofuranosyl]-3′-spiro-5″-(4″-amino--oxathiole--dioxide)thymine derivatives as inhibitors of HIV-1 reverse transcriptase dimerization. J. Med. Chem. 2006;49:4834–4841. doi: 10.1021/jm0604575. [DOI] [PubMed] [Google Scholar]
- 140.Smee D.F., Wong M.-H., Bailey K.W., Beadle J.R., Hostetler K.Y., Sidwell R.W. Effects of four antiviral substances on lethal vaccinia virus (IHD strain) respiratory infections in mice. Int. J. Antimicrob. Agents. 2004;23:430–437. doi: 10.1016/j.ijantimicag.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 141.Snoeck R. Papillomavirus and treatment. Antiviral Res. 2006;71:181–191. doi: 10.1016/j.antiviral.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 142.Snoeck R., Andrei G., Bodaghi B., Lagneaux L., Daelemans D., De Clercq E., Neyts J., Schols D., Naesens L., Michelson S., Bron D., Otto M.J., Bousseau A., Nemecek C., Roy C. 2-Chloro-3-pyridin-3-yl-5,6,7,8-tetrahydroindolizine-1-carboxamide (CMV423), a new lead compound for the treatment of human cytomegalovirus infections. Antiviral Res. 2002;55:413–424. doi: 10.1016/s0166-3542(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 143.Soriano V., Puoti M., Bonacini M., Brook G., Cargnel A., Rockstroh J., Thio C., Benhamou Y. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV international panel. AIDS. 2005;19:221–240. [PubMed] [Google Scholar]
- 144.Spaltenstein A., Kazmierski W.M., Miller J.F., Samano V. Discovery of next generation inhibitors of HIV protease. Curr. Topics Med. Chem. 2005;5:1589–1607. doi: 10.2174/156802605775009694. [DOI] [PubMed] [Google Scholar]
- 145.Stittelaar K.J., Neyts J., Naesens L., van Amerongen G., van Lavieren R.F., Holý A., De Clercq E., Niesters H.G.M., Fries E., Maas C., Mulder P.G.H., van der Zeijst B.A.M., Osterhaus A.D.M.E. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 146.Strizki J.M., Tremblay C., Xu S. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2005;49:4911–4919. doi: 10.1128/AAC.49.12.4911-4919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takashima K., Miyake H., Kanzaki N., Tagawa Y., Wang X., Sugihara Y., Iizawa Y., Baba M. Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small-molecule CCR5 antagonist. Antimicrob. Agents Chemother. 2005;49:3474–3482. doi: 10.1128/AAC.49.8.3474-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang S., Tan M., McCoy J.P., Jr, Zheng Z.M. Short-term induction and long-term suppression of HPV16 oncogene silencing by RNA interference in cervical cancer cells. Oncogene. 2006;25:2094–2104. doi: 10.1038/sj.onc.1209244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tremblay C.L., Giguel F., Guan Y., Chou T.-C., Takashima K., Hirsch M.S. TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti-human immunodeficiency virus interactions with other antiretrovirals in vitro. Antimicrob. Agents Chemother. 2005;49:3483–3485. doi: 10.1128/AAC.49.8.3483-3485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Bömmel F., Wünsche T., Mauss S., Reinke P., Bergk A., Schurmann D., Wiedenmann B., Berg T. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–1425. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 151.van Bömmel F., Zöllner B., Sarrazin C., Spengler U., Hüppe D., Möller B., Feucht H.-H., Wiedenmann B., Berg T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318–325. doi: 10.1002/hep.21253. [DOI] [PubMed] [Google Scholar]
- 152.Vermeire K., Princen K., Hatse S., De Clercq E., Dey K., Bell T.W., Schols D. CADA, a novel CD4-targeted HIV inhibitor, is synergistic with various anti-HIV drugs in vitro. AIDS. 2004;18:2115–2125. doi: 10.1097/00002030-200411050-00003. [DOI] [PubMed] [Google Scholar]
- 153.Watson C., Jenkinson S., Kazmierski W., Kenakin T. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol. Pharmacol. 2005;67:1268–1282. doi: 10.1124/mol.104.008565. [DOI] [PubMed] [Google Scholar]
- 154.Westby M., van der Ryst E. CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection. Antiviral Chem. Chemother. 2005;16:339–354. doi: 10.1177/095632020501600601. [DOI] [PubMed] [Google Scholar]
- 155.White P.W., Faucher A.-M., Massariol M.-J., Welchner E., Rancourt J., Cartier M., Archambault J. Biphenylsulfonacetic acid inhibitors of the human papillomavirus type 6 E1 helicase inhibit ATP hydrolysis by an allosteric mechanism involving tyrosine 486. Antimicrob. Agents Chemother. 2005;49:4834–4842. doi: 10.1128/AAC.49.12.4834-4842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu T., Froeyen M., Kempeneers V., Pannecouque C., Wang J., Busson R., De Clercq E., Herdewijn P. Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents. J. Am. Chem. Soc. 2005;127:5056–5065. doi: 10.1021/ja043045z. [DOI] [PubMed] [Google Scholar]
- 157.Wursthorn K., Lutgehetmann M., Dandri M., Volz T., Buggisch P., Zollner B., Longerich T., Schirmacher P., Metzler F., Zankel M., Fischer C., Currie G., Brosgart C., Petersen J. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HbsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 158.Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., De Clercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang H., Kim S.-K., Kim P.A., Reche M., Morehead T.J., Damon I.K., Welsh R.M., Reinherz E.L. Antiviral chemotherapy facilitates control of poxvirus infections through inhibition of cellular signal transduction. J. Clin. Invest. 2005;115:379–387. doi: 10.1172/JCI23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yates P.J., Hazen R., St. Clair M., Boone L., Tisdale M., Elston R.C. In vitro development of resistance to human immunodeficiency virus protease inhibitor GW640385. Antimicrob. Agents Chemother. 2006;50:1092–1095. doi: 10.1128/AAC.50.3.1092-1095.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yatsuji H., Noguchi C., Hiraga N., Mori N., Tsuge M., Imamura M., Takahashi S., Iwao E., Fujimoto Y., Ochi H., Abe H., Maekawa T., Tateno C., Yoshizato K., Suzuki F., Kumada H., Chayama K. Emergence of a novel lamivudine-resistant hepatitis B virus variant with a substitution outside the YMDD motif. Antimicrob. Agents Chemother. 2006;50:3867–3874. doi: 10.1128/AAC.00239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yeo H., Li Y., Fu L., Zhu J.L., Gullen E.A., Dutschman G.E., Lee Y., Chung R., Huang E.S., Austin D.J., Cheng Y.C. Synthesis and antiviral activity of helioxanthin analogues. J. Med. Chem. 2005;48:534–546. doi: 10.1021/jm034265a. [DOI] [PubMed] [Google Scholar]
- 163.Ying C., De Clercq E., Neyts J. Selective inhibition of hepatitis B virus replication by RNA interference. Biochem. Biophys. Res. Commun. 2003;309:482–484. doi: 10.1016/j.bbrc.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 164.Yu D., Sakurai Y., Chen C.H., Chang F.R., Huang L., Kashiwada Y., Lee K.H. Anti-AIDS agents 69, Moronic acid and other triterpene derivatives as novel potent anti-HIV agents. J. Med. Chem. 2006;49:5462–5469. doi: 10.1021/jm0601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zeng M., Mao Y., Yao G., Wang H., Hou J., Wang Y., Ji B.N., Chang C.-N.P., Barker K.F. A double-blind randomized trial of adefovir dipivoxil in Chinese subjects with HBeAg-positive chronic hepatitis B. Hepatology. 2006;44:108–116. doi: 10.1002/hep.21225. [DOI] [PubMed] [Google Scholar]
- 166.Zhou S., Breitenbach J.M., Borysko K.Z., Drach J.C., Kern E.R., Gullen E., Cheng Y.C., Zemlicka J. Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines: second-generation methylenecyclopropane analogues of nucleosides. J. Med. Chem. 2004;47:566–575. doi: 10.1021/jm030316s. [DOI] [PubMed] [Google Scholar]
- 167.Zhou S., Kern E.R., Gullen E., Cheng Y.-C., Drach J.C., Tamiya S., Mitsuya H., Zemlicka J. 9-{[3-Fluoro-2-(hydroxymethyl)cyclopropylidene]methyl}-adenines and -guanines. Synthesis and antiviral activity of all stereoisomers. J. Med. Chem. 2006;49:6120–6128. doi: 10.1021/jm0607404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou X.-J., Fielman B.A., Lloyd D.M., Chao G.C., Brown N.A. Pharmacokinetics of telbivudine in healthy subjects and absence of drug interaction with lamivudine or adefovir dipivoxil. Antimicrob. Agents Chemother. 2006;50:2309–2315. doi: 10.1128/AAC.01313-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu W., Burnette A., Dorjsuren D., Roberts P.E., Huleihel M., Shoemaker R.H., Marquez V.E., Agbaria R., Sei S. Potent antiviral activity of north-methanocarbathymidine against Kaposi's sarcoma-associated herpesvirus. Antimicrob. Agents Chemother. 2005;49:4965–4973. doi: 10.1128/AAC.49.12.4965-4973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zoulim F. Entecavir: a new treatment option for chronic hepatitis B. J. Clin. Virol. 2006;36:8–12. doi: 10.1016/j.jcv.2006.01.010. [DOI] [PubMed] [Google Scholar]


