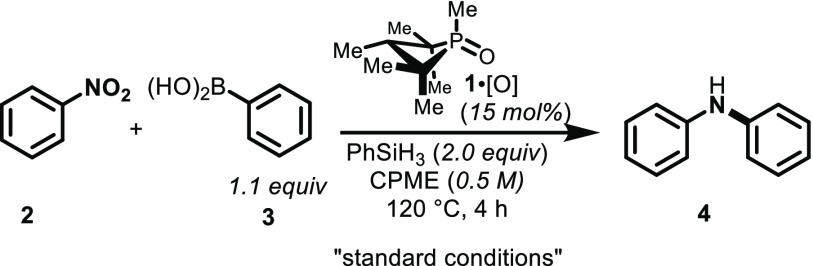
Table 1. Effect of Hydrosilane Loading and Identity on the Organophosphorus-Catalyzed Reductive C–N Coupling Reaction.
| entry | change from “standard conditions” | conv (yield) (%)a |
|---|---|---|
| 1 | none | 99 (96) |
| 2 | 5 mol% of 1·[O], 10 h | 99 (95) |
| 3 | 2 mol% of 1·[O], 36 h | 99 (93) |
| 4 | 80 °C, 20 h | 99 (95) |
| 5 | 60 °C, 96 h | 99 (93) |
| 6 | 0.77 equiv of PhSiH3 | 98 (94) |
| 7 | 0.66 equiv of PhSiH3 | 85 (79) |
| 8 | 0.33 equiv of PhSiH3 | 49 (46) |
| 9 | 3.0 equiv of Ph2SiH2 | 96 (88) |
| 10 | 3.0 equiv of TMDSc | 93 (85) |
| 11 | 1.5 equiv of TMCTSb | 99 (83) |
| 12 | 4.0 equiv of PMHS | 99 (96) |
| 13 | Ph-Bpin instead of PhB(OH)2 | 49 (trace) |
Yields were determined through analysis by gas chromatography with the use of dodecane as an internal standard.
TMCTS = 2,4,6,8-tetramethylcyclotetrasiloxane.
TMDS = 1,1,3,3-tetramethyldisiloxane.