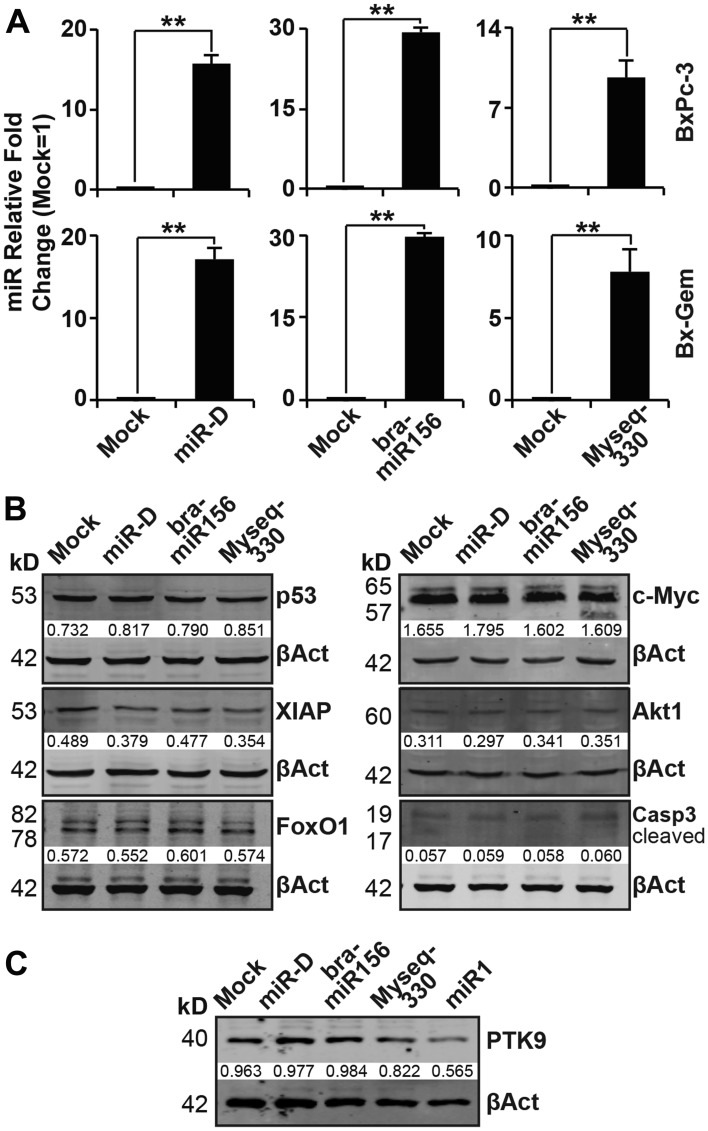
Figure 3. Lipofection of broccoletti-miRs in PDA cells does not induce the expression of target genes.
(A) BxPc-3 and Bx-Gem cells were transfected with bra-miR156g-5p, Myseq-330, miR-D (50 nM each), or a miR-NG oligonucleotide (50 nM), which served as a mock control. At 24 h after transfection, the RNA was isolated, followed by reverse transcription. The samples were validated by TaqMan® miR assay. RNU44 was used to normalize the expression level, and the average fold change of the mock control was set to 1. The relative fold changes ×1000 are presented. Experiments were performed in triplicate, and the data are shown as the means ± SD (** P < 0.01). (B) Proteins were harvested from BxPc-3 cells at 24 h after lipofection, and western blot analysis was performed for p53, XIAP, FoxO1, c-Myc, Akt1 and caspase-3. β-actin served as a control for equal loading conditions. The protein sizes are shown in kilodaltons (kD). The band intensities were measured using ImageJ and are shown below the bands. The band intensities were normalized to β-actin. (C) Total proteins were harvested from BxPc-3 cells at 24 h after lipofection, and PTK9 protein levels were evaluated via western blot analysis and examined as described above.