FIG. 1.
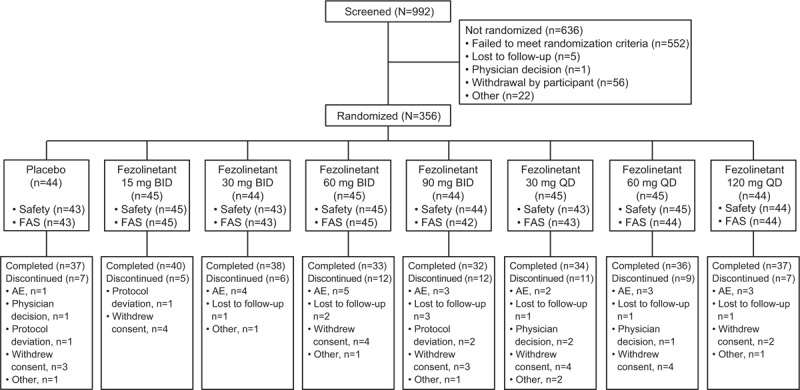
Participant disposition. Safety included all participants who were randomized and received at least one dose of study medication. FAS included all randomized participants who received at least one dose of study drug and had baseline and postbaseline efficacy evaluation. AE, adverse event; FAS, full analysis set.
