Fig 3. Cumulative survival rates of 69 patients with unresectable hepatocellular carcinoma (HCC) receiving lenvatinib as evaluated using the Kaplan-Meier method.
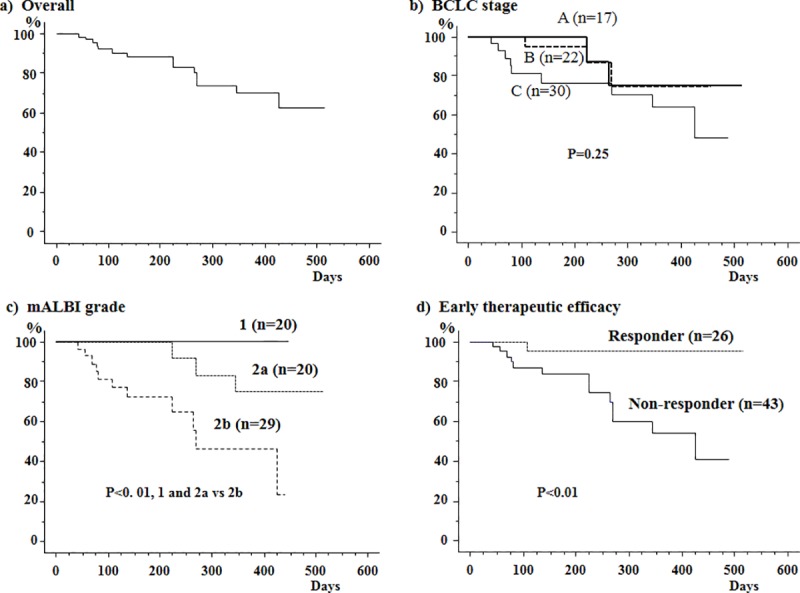
a) The cumulative overall survival rates after the initiation of lenvatinib were 88.5% at 24 weeks and 73.9% at 48 weeks. b) The rates did not differ between patients with Barcelona Clinic Liver Cancer (BCLC) stages A and B HCC and those with stage C HCC. c) The rates in patients with modified albumin-bilirubin (mALBI) grade 1 and grade 2a at baseline were 90.9% at 48 weeks; these values were significantly higher than the rate in patients with mALBI grade 2b at baseline (46.6%) (P<0.01). d) The rates differed depending on the early therapeutic efficacies; the rates at 24 and 48 weeks were significantly higher in patients achieving CR and PR (95.5% and 95.5%, respectively) than in those showing no response (84.0% and 54.3%, respectively) (P<0.01).
