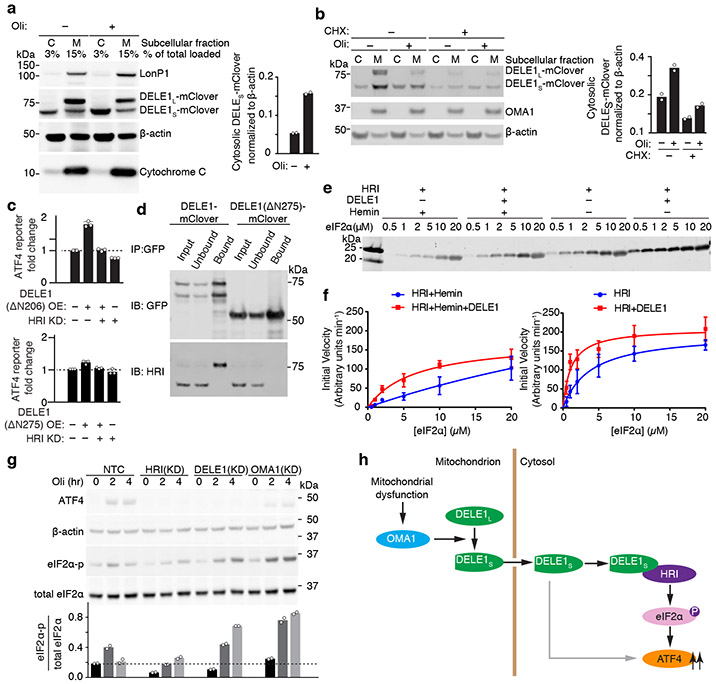
Fig. 4. Cytosolic DELE1 physically interacts with and activates HRI.
(a) Immunoblot of biochemical fractionations of cells stably expressing DELE1-mClover treated with 1.25 ng/mL oligomycin (Oli) for 16 h where indicated. Left, representative immunoblot of the cytosolic (C) and mitochondrial (M) fractions. LonP1 and cytochrome c were probed as mitochondrial markers. Right, quantification of the cytosolic DELE1S-mClover from n = 2 blots.
(b) Accumulation of DELE1S is protein-synthesis independent. Biochemical fractionation of cells stably expressing DELE1-mClover treated with 1.25 ng/mL oligomycin (Oli) and 20 μg/mL cycloheximide (CHX) for 4 h where indicated. Left, representative immunoblot of the cytosolic (C) and mitochondrial (M) fractions. OMA1 as probed as mitochondrial marker. Right, quantification of the cytosolic DELE1S-mClover from n = 2 blots.
(c) Transient overexpression (OE) of the cytosolically localized DELE1(ΔN206), but not the DELE1(ΔN275) construct induces the ATF4 reporter. Knockdown (KD) of HRI blocks reporter activation (mean ± s.d., n = 3 culture wells).
(d) Co-immunoprecipitation of HRI with transiently expressed full-length DELE1-mClover but not with DELE1(ΔN275)-mClover. Similar results in n = 2 independent experiments.
(e,f) HRI enzyme kinetics in the presence or absence of DELE1 with or without 5 μM hemin using different concentrations of the substrate eIF2α. (e) Representative immunoblot. (f) Quantification and fitting to the Michaelis-Menten equation (mean ± s.e.m., n = 3 independent experiments).
(g) Top, immunoblot of ATF4, phospho-eIF2α, total eIF2α in NTC control, HRI KD-, DELE1 KD- and OMA1 KD-cells treated with 1.25ng/mL oligomycin for 0, 2 and 4 hrs. Bottom, quantification of the phospho-eIF2α to total eIF2α ratio from n = 2 blots.
(h) Model.