Abstract
Commercially-available assays utilizing antigen or nucleic acid detection chemistries provide options for mosquito control districts to screen their mosquito populations for arboviruses and make timely operational decisions regarding vector control. These assays may be utilized even more advantageously when combined with honey-soaked nucleic acid preservation substrate (“honey card”) testing by reducing or replacing the time- and labor-intensive efforts of identifying and processing mosquito pools. We tested artificially inoculated honey cards and cards fed upon individually by West Nile virus (WNV) and Zika virus (ZIKV) infected mosquitoes with three assays to compare detection rates and the limit of detection for each platform with respect to virus detection of a single infected mosquito, and quantify the time-interval of virus preservation on the cards. Assays evaluated included CDC protocols for real-time RT-PCR for WNV and ZIKV, Pro-Lab Diagnostics ProAmpRT™ WNV loop mediated amplification (LAMP) and ZIKV LAMP assays, and the Rapid Analyte Measurement Platform (RAMP) WNV assay. Real time RT-PCR was the most sensitive assay and the most robust to viral RNA degradation over time. To maximize the detection of virus, honey cards should be left in the traps ≤ 1 day if using LAMP assays, ≤ 3 days if using real time RT-PCR to detect viruses from field samples. The WNV RAMP assay, although effective for pool-screening, lacks sensitivity required for honey card surveillance. Future studies may determine the minimum number of infectious mosquitoes required to feed on a honey card that would be reliably detected by the LAMP or RAMP assays.
Keywords: RAMP® test, West Nile Virus, Zika virus, honey cards, arbovirus surveillance, mosquito, ProAmpRT™ LAMP assay
INTRODUCTION
Mosquito-transmitted pathogens such as West Nile virus (WNV) and Zika virus (ZIKV) pose imminent public health threats to the United States (US). Early detection of infected mosquitoes is critical to direct timely vector-control measures or to recommend strategies for disease prevention. Pool-based arbovirus surveillance typically involves the collection, species identification, pooling, and processing of mosquito specimens prior to testing, which is a time- and labor-intensive process, requiring resources that are limited in many areas. A relatively new technique initially developed by Australian researchers for the surveillance of arboviruses in remote areas of the country (Hall-Mendelin et al. 2010) show promise as an alternative or a supplement to pool-based arbovirus screening testing in the US. Honey-infused nucleic acid-preserving substrates (“honey cards”) are placed within collection chambers of modified mosquito traps (Ritchie et al. 2013, Johnson et al. 2015, Burkett-Cadena et al. 2016) or traditional encephalitis virus surveillance (EVS) traps (Flies et al. 2015) so that trapped mosquitoes within the chambers feed upon the honey for their nourishment and an infectious mosquito will deposit virus particles onto the honey card along with the saliva as it feeds. The virus is inactivated and preserved by chemicals impregnated within the honey cards (Hall-Mendelin et al. 2010). The honey cards are removed from the traps and tested for arboviruses using molecular methods such as real time reverse transcriptase polymerase chain reaction (RT-PCR), which permits the screening of large numbers of mosquitoes without the laborious tasks of handling, identifying to species, and processing them, as well as eliminating the need to maintain a cold chain as cards are transported from the field to the laboratory. Additionally, this procedure targets only infectious mosquitoes transmitting arboviruses by bite, which provides the most useful information for the public health goals of arbovirus surveillance and cannot be determined through traditional pool-based testing methods.
Although real-time RT-PCR is a highly sensitive and target-specific method to screen for arboviruses, it requires specialized technical expertise to perform and the instruments and reagents are expensive. In this study, we evaluated commercially available assays that were originally developed for mosquito pool-based arbovirus surveillance as alternatives to real time RT-PCR to assess their potential utility in the honey card surveillance method.
Three assays were used to evaluate WNV samples: the Rapid Analyte Measurement Platform (RAMP®) WNV test (Response Biomedical Corp., Burnaby, British Columbia, Canada), the ProAmpRT™ WNV loop mediated amplification (LAMP) assay (Pro-Lab Diagnostics, Round Rock, TX) and real time RT-PCR (Lanciotti et al. 2000). The RAMP WNV test is a lateral-flow assay used to detect WNV antigen in a sample. The LAMP assay is an isothermal nucleic acid detection assay that amplifies specific regions of a target gene (Notomi et al. 2000). Reverse transcriptase added to the reaction allows the amplification and detection of RNA.
Two assays were used to evaluate ZIKV samples: the ProAmpRT™ ZIKV LAMP assay and real-time RT-PCR (Lanciotti et al. 2007). With the exception of primers that specifically target ZIKV and a longer amplification time, the ProAmpRT ZIKV LAMP assay is identical to the WNV assay in mechanism and protocol. At the time of writing, a RAMP ZIKV assay was not available.
In this evaluation, we tested honey cards artificially inoculated with WNV and ZIKV with commercial testing platforms designed for these specific viral targets to determine the limit of detection of these assays, optimize honey card processing protocols, and determine the persistence of viral RNA on honey cards for up to 7 days under mock-field conditions. In order to determine the ability of the assays to detect expectorated virus in a trap with infected mosquitoes, honey cards fed upon by individual WNV- and ZIKV-infected mosquitoes were also tested to determine if these assays could detect viral RNA expectorated by a single virus-positive mosquito.
METHODS
Virus isolates and propagation
A 2015 Puerto Rico isolate of ZIKV from a human clinical sample (Asian lineage, GenBank accession: KU501215.1) was provided by the Centers for Disease Control and Prevention (CDC). This strain of ZIKV was chosen for its role in Zika fever outbreaks in the Americas (December 2015-present) (Lanciotti et al. 2016) and it is similar to ZIKV isolates introduced in Florida (Grubaugh et al. 2017, Metsky et al. 2017). A 2003 isolate of WNV (GenBank accession: DQ983578) from Indian River County, Florida was retrieved from the archives at the Florida Medical Entomology Laboratory (FMEL). Viral stocks were propagated in cultured African green monkey (Vero) cells and viral titer determined by plaque assay using procedures similar to established techniques (Faye et al. 2013, Alto et al. 2014, Kaur et al. 2016). Low passage (< 5 passages) virus cultures were combined with defibrinated bovine blood or chicken blood with Alsever’s solution (Hemostat Laboratories, Dixon, CA) and ATP (0.005 M) and used for the oral infection of mosquitoes.
Mosquito infection
Established laboratory colonies of Culex quinquefasciatus (F>50 generations from Indian River Co., and Alachua Co., FL) and Aedes aegypti (F1-F3 generations from Martin Co., FL) were used in the WNV and ZIKV infection studies, respectively. Adult females (10–11 days old) were placed in cages with mesh screening and allowed to feed for one hour on ZIKV- or WNV-infected blood using an artificial feeding system (Hemotek, Lancashire, United Kingdom) with hog casing membranes. We used a dose-response study consisting of a high dose to maximize infection and transmission potential rates, and a lower dose representing WNV viremia profiles in infected birds (Komar et al. 2003; Guerrero-Sánchez et al. 2011) and ZIKV viremia profiles in infected humans (Lanciotti et al. 2008). Aliquots of blood were stored at −80°C for later determination of virus titer by plaque assay (Alto et al. 2014, Faye et al. 2013). Mosquitoes were exposed to 8.1 – 8.4 (high) and 5.6 – 8.0 (low) log10 plaque forming units (PFU)/ml of WNV and 6.2–6.3 (high) and 5.1–5.3 (low) log10 PFU/ml of ZIKV for the experiments with Cx. quinquefasciatus and Ae. aegypti, respectively. After the feeding trials, fully engorged females were held in cages along with an oviposition substrate and maintained at a 14:10 hour light:dark photoperiod at 30°C for 13 days, after which individual mosquitoes were placed in 37-mL plastic tubes (height by diameter: 8cm by 3cm). Each tube held one female mosquito that was presented with a honey-soaked University-Storage and Transport Optimized Platform (U-STOP) card (referred to as a “honey card”) fastened to the inside of the lid fitted with a removable screen (Fig. 1). Undiluted honey was dyed with blue food coloring to provide a visual marker indicating that a mosquito fed on the honey and expectorated saliva onto the cards (Burkett-Cadena et al. 2016, Alto et al. 2017). Honey was added to cards approximately 24–72 hours before use and kept at 4°C. Mosquitoes were examined using a flashlight for blue in their crop during the transmission experiment. Mosquitoes that did not feed on the blue honey were excluded from further analysis of WNV and ZIKV transmission. Mosquitoes and honey cards were checked at 24 and 48 hours and collected upon detection of blue in the crop; therefore, saliva with virus remained on cards no longer than 24 hours before removal from the tubes. Mosquitoes were frozen at −80°C until tested as described below. Honey cards were held in incubators approximating field conditions (28°C at 60–80% relative humidity (RH)) and removed for testing at 1, 3 and 7 days. Mosquitoes and honey cards were shipped to the CDC in Fort Collins, CO for WNV and ZIKV testing as described below.
Fig 1.

After oral infection of West Nile virus (WNV) or Zika virus (ZIKV), individual mosquitoes were held in tubes and presented with a honey-soaked University-Storage and Transport Optimized Platform (U-STOP) card dyed with blue food coloring to indicate when a mosquito had fed and deposited saliva onto the card. Mosquitoes that were observed with blue in their crop (inset) and their associated honey cards were removed for virus testing.
Arbovirus inoculation of honey cards
To determine the sensitivity of the three detection platforms, honey cards were inoculated with one of three doses of virus applied to the card in a 67.5 μl volume. We used three doses of WNV (2.8, 3.8, and 4.8 log10 PFU) and ZIKV (3.6, 4.6, and 5.6 log10 PFU) ZIKV. Cards were held in tubes in an incubator at 28°C and 60–80% RH and removed at 1, 3 and 7 days and placed at −80°C until tested as described below.
Molecular detection
To determine the virus dissemination status of mosquitoes, legs were removed from each mosquito before processing and the bodies and associated legs were placed in separate 2 ml flat-bottomed tubes. Bodies and legs were homogenized by vortex with one 4.4 mm steel shot (BB) in 1 ml or 500 μl cell culture medium bovine albumin (BA)-1, respectively, and centrifuged at 1700 x g for 3 min. A 100-μl aliquot of supernatant was extracted from each mosquito sample for viral RNA with a Qiagen BioRobot Universal instrument (QIAGEN, Inc., Valencia, CA) using Qiagen’s QIAamp Virus BioRobot 9604 kit according to the manufacturer’s instructions.
Mosquito-fed honey cards and 5 replicates of artificially inoculated honey cards were extracted for viral RNA using Qiagen’s QIAamp Viral RNA Mini Kit. Variations on extraction protocols, including vortexing the cards in BA-1 prior to removing an aliquot for extraction, were compared on a subset of inoculated cards to select the extraction procedure used in this evaluation that provided the most sensitive real-time RT-PCR results (data not shown). Each honey card was incubated in 800 μl lysis buffer AL for one hour, vortexed for 1–2 minutes every 20 minutes, and centrifuged as described above. A 500 μl aliquot of the lysis buffer mixture was added to an appropriate amount of ethanol and extracted for viral RNA according to the manufacturer’s instructions.
Real-time RT-PCR was performed on RNA extracted from the mosquito bodies, legs, and honey cards using Qiagen’s QuantiTect Probe RT-PCR Kit and primers targeting either WNV (Lanciotti et al. 2000) or ZIKV (Lanciotti et al. 2008). Samples returning a cycle threshold (CT) value of ≤ 38 were considered putatively positive. RT-LAMP assays were performed on RNA extracted from honey cards using the ProAmpRT™ WNV and ProAmpRT™ ZIKAV assays, which contain reagents and a fluorescently-labeled primer mix specific to WNV or ZIKV that are added to extracted RNA. The reactions were carried out on a preprogrammed OptiGene Genie II instrument (OptiGene, Horsham, United Kingdom) which measures emitted fluorescence from the samples; positive samples are characterized by an annealing temperature specific to the target gene (~87–88°C) as determined by the manufacturer.
The RAMP WNV assay was used to test 2 replicates of each inoculation dose and incubation period of the honey cards artificially inoculated with WNV. One mL RAMP buffer was added to each card and incubated for one hour, and the homogenization protocol was followed as described above. According to the manufacturer’s instructions, an aliquot of supernatant was applied to an immunochromatographic strip housed in a cartridge. After a 90-minute incubation, the cartridge was read by the RAMP Reader that displays the results in RAMP units. RAMP unit results of ≥ 50 were considered positive (Burkhalter et al. 2014). Based on the results from the inoculated cards, the RAMP WNV assay was excluded from further evaluation of the mosquito-fed honey cards.
Statistical analysis
GraphPad Prism software was used to analyze the data. A one-way analysis of variance (ANOVA) was used to determine significant differences (p < 0.05) in mean CT values of artificially inoculated honey cards after incubation for 1, 3, or 7 days. Statistically significant differences were calculated using post-hoc Student t-tests to further analyze mean CT differences between time points.
RESULTS
Arbovirus inoculated honey cards
WNV-inoculated honey cards produced positive real time RT-PCR results (CT ≤ 38) for all 5 replicates of titers (2.8, 3.8 and 4.8 log10 PFU) and time points (1, 3, and 7 days) (Fig 2). For cards inoculated with 3.8 or 4.8 log10 PFU WNV, there were no statistically significant differences between means at days 1, 3, or 7 (3.8 log10 PFU: F2,12 = 1.17, p = 0.34; 4.8 log10 PFU/ml: F2,11 = 1.88, p = 0.20). Cards inoculated with 2.8 log10 PFU produced statistically significantly higher CT values when incubated for 7 days compared to cards incubated for 1 or 3 days (F2,12 = 8.59, p = 0.005). LAMP results for the WNV-inoculated cards are found in Table 1. All cards (5/5) inoculated with the highest dose (4.8 log10 PFU) and held for 1 or 3 days were positive by the LAMP assay; by day 7, 4/5 replicates inoculated with the highest dose were detectable. Four of 5 cards inoculated with 2.8 and 3.8 log10 PFU after 1 day of incubation were positive by LAMP; longer incubation periods resulted in fewer positive cards detected. None of the WNV inoculated cards produced positive results (≥ 50) in the RAMP WNV assay.
Fig 2.
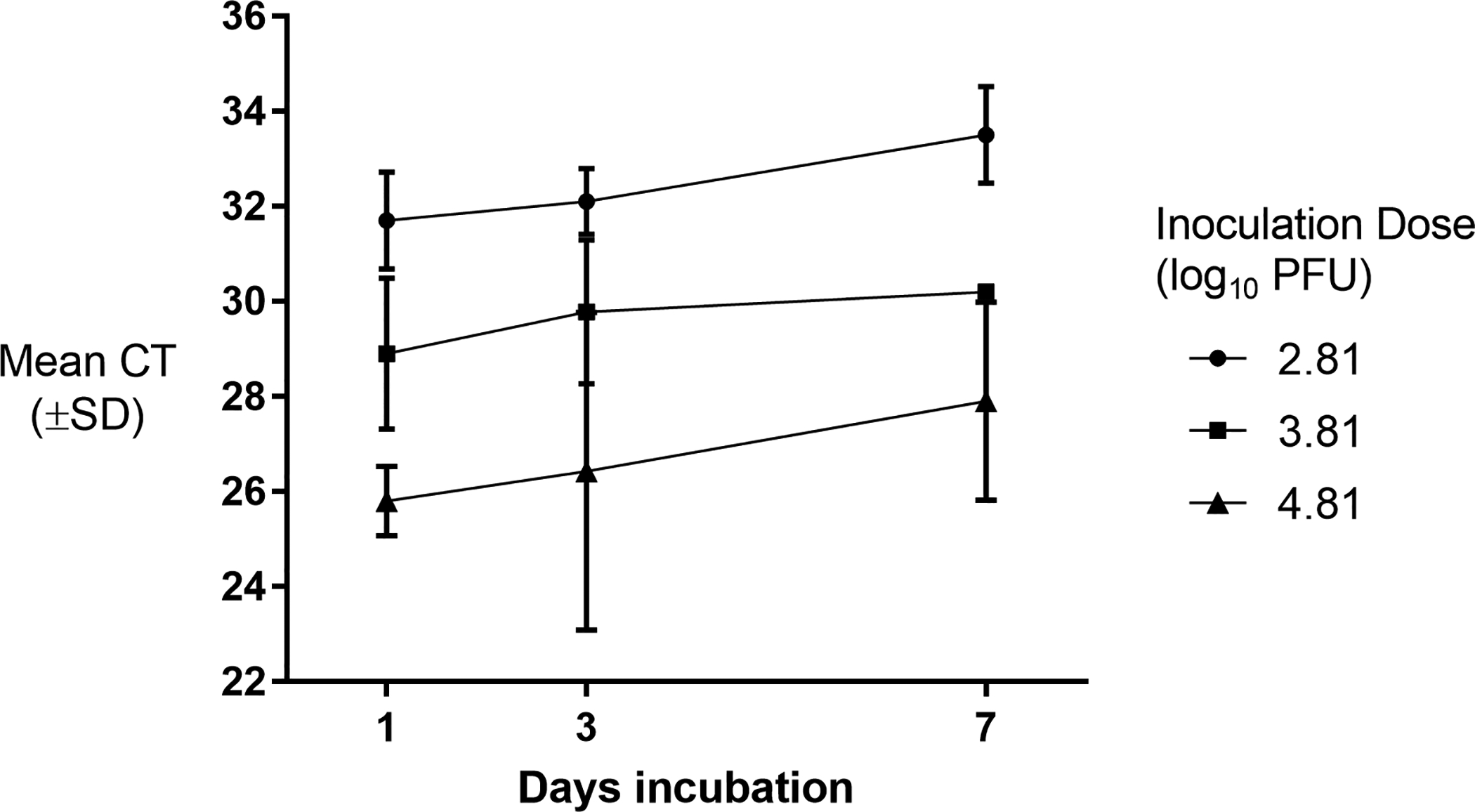
Real time RT-PCR detection of West Nile virus (WNV) RNA from artificially inoculated honey cards (n = 5) after incubation for 1, 3, and 7 days at 28°C and 60–80% RH. The inoculation dose is expressed in plaque forming units (PFU).
Table 1.
Loop-mediated amplification (LAMP) detection of West Nile virus (WNV) and Zika virus (ZIKV) RNA from artificially inoculated honey cards (n = 5) after incubation for 1, 3, and 7 days at 28°C and 60–80% relative humidity. The inoculation dose is expressed in plaque forming units (PFU).
| No. positive cards per days incubation | ||||
|---|---|---|---|---|
| Inoculation titer (PFU) | Day 1 | Day 3 | Day 7 | |
| WNV | 2.8 | 4 (80%) | 3 (60%) | 3 (60%) |
| 3.8 | 4 (80%) | 3 (60%) | 2 (40%) | |
| 4.8 | 5 (100%) | 5 (100%) | 4 (80%) | |
| ZIKV | 3.6 | 5 (100%) | 2 (40%) | 0 (0%) |
| 4.6 | 4 (80%) | 3 (60%) | 2 (40%) | |
| 5.6 | 5 (100%) | 4 (80%) | 2 (40%) | |
ZIKV-inoculated cards also produced positive real time RT-PCR results (CT ≤ 38) at all 5 replicates of titers (3.6, 4.6 and 5.6 log10 PFU) and time points (Fig 3). Cards inoculated with 4.6 and 5.6 log10 PFU and held for 3 or 7 days produced statistically significantly higher mean CTs when compared to cards held for 1 day (4.6 log10 PFU: (F2,12 = 9.73, p = 0.003; 5.6 log10 PFU: (F2,12 = 4.24, p = 0.04). There was no statistically significant difference in mean CTs in cards inoculated with 3.6 log10 PFU/ml at any time point (F2,11 = 1.81, p = 0.21). LAMP results for the ZIKV-inoculated cards are found in Table 1. For all titers, the number of replicates detectable by LAMP decreased as incubation periods increased. After 7 days incubation, few cards (2/5) inoculated with 4.6 and 5.6 log10 PFU and none of the cards inoculated with 3.6 log10 PFU were detectable by the LAMP assay.
Fig 3.
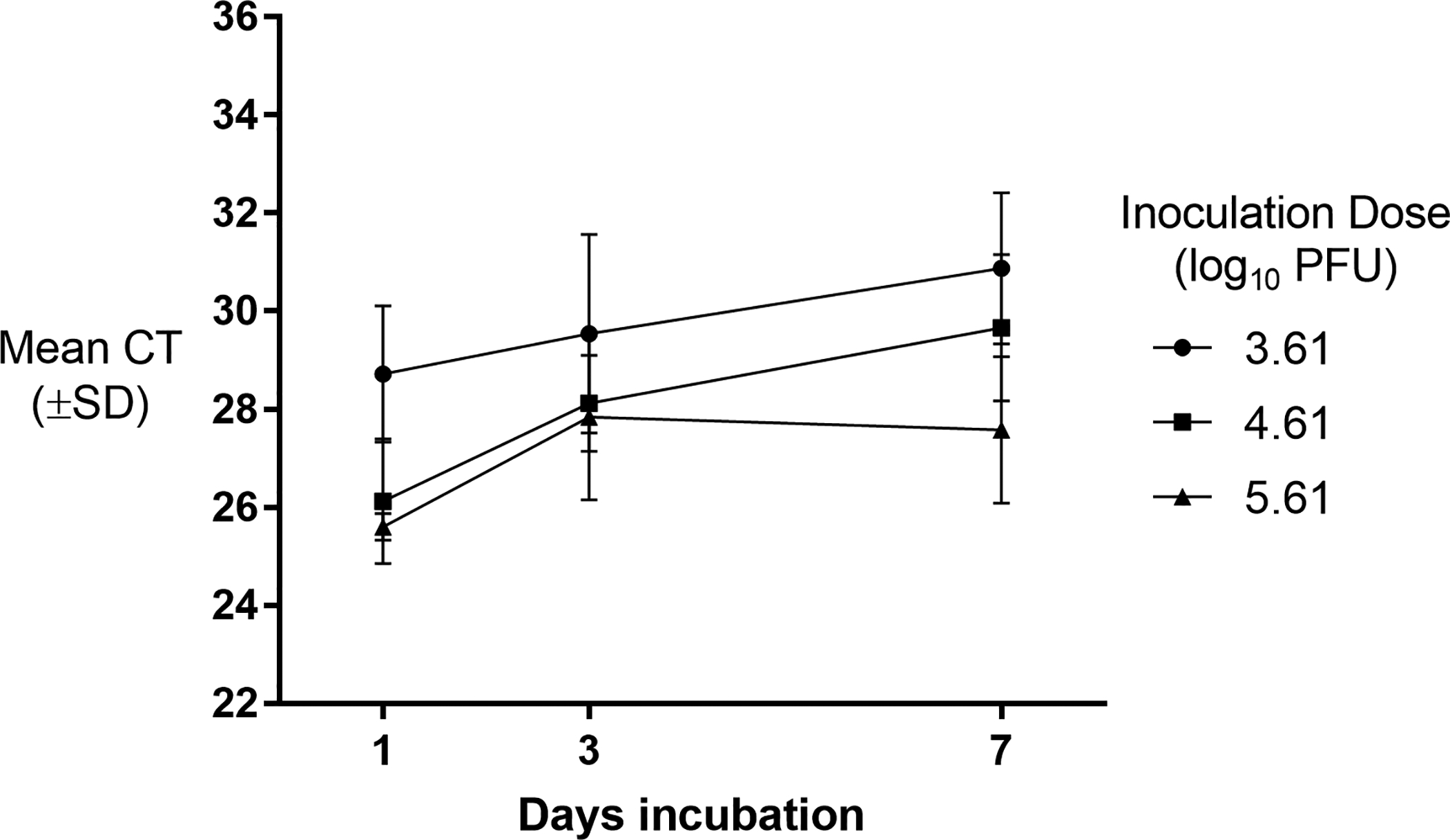
Real time RT-PCR detection of Zika virus (ZIKV) RNA from artificially inoculated honey cards (n = 5) after incubation for 1, 3, and 7 days at 28°C and 60–80% RH. The inoculation dose is expressed in plaque forming units (PFU).
Infected mosquitoes
Of the 25 mosquitoes that had disseminated WNV infections and individually fed on honey cards (indicated by the presence of blue dye in their crop), a total of 18 (72%) expectorated virus that was detectable on the honey cards by real time RT-PCR (Table 2). By time point, 62.5% (5/8), 80% (8/10), and 71.4% (5/7) of cards held for 1, 3, or 7 days, respectively, were detectable by real time RT-PCR. There was no significant difference in mean CTs of cards collected on days 1, 3, or 7 (F2,15 = 0.15, p = 0.861). Two of the 25 (8%) infected mosquitoes with disseminated infections expectorated virus that was detectable by the LAMP assay, both detected on cards held for 3 days.
Table 2.
Real time RT-PCR and Loop-mediated amplification (LAMP) viral RNA detection from honey cards fed upon by mosquitoes with disseminated West Nile virus (WNV; n = 25) and Zika virus (ZIKV; n = 12) infections. Cards were incubated for 1, 3, or 7 days at 28°C and 60–80% relative humidity.
| No. cards positive by real-time RT-PCR | No. cards positive by LAMP | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Total cards | Day 1 | Day 3 | Day 7 | Total cards | |
| Mean CT (SD) | 34.9 (1.49) | 34.8 (1.96) | 34.1 (3.35) | N/A | N/A | N/A | N/A | N/A |
| Mean CT (SD) | 37.4 (.028) | 32.6 (0.85) | N/A | N/A | N/A | N/A | N/A | N/A |
Of the 12 ZIKV-positive mosquitoes that had disseminated infections and fed on honey cards, a total of 4 (33.3%) expectorated virus that was detectable by real time RT-PCR (Table 2). The mean CTs of cards held for 1 day were statistically significantly higher than cards held for 3 days (p = 0.008). No virus was detected in cards held for 7 days by real time RT-PCR. The ZIKA LAMP assay did not detect virus from any of the mosquito-fed honey cards.
DISCUSSION
While arbovirus surveillance is an important tool for the control of human pathogens, traditional mosquito pool-based surveillance requires significant resources to collect, process, and screen insects for arboviruses. Honey-card based arbovirus screening protocols were developed in Australia, where it is often necessary to deploy traps in remote areas that are difficult to access frequently to collect and screen the trapped mosquitoes (van den Hurk et al. 2014). For typical arbovirus surveillance programs in the US, we submit that the primary advantage of this technique is reducing the testing load from many mosquito pools per trap to one or two honey cards per trap. Previous evaluations of this technique have described successful arbovirus detection from honey cards fed upon by several mosquitoes using real time RT-PCR (Lothrop et al. 2012, Flies et al. 2015, Burkett-Cadena et al. 2016). Our evaluation here assessed the potential utility of commercially available assays, in addition to real time RT-PCR, as options for honey card-based arbovirus surveillance. To that end we investigated ability of these assays to detect virus expectorated onto a honey card from a single positive mosquito, and their ability to detect known quantities of WNV or ZIKV RNA deposited on the cards and incubated over several days under field-like conditions.
First, we evaluated the assays’ limit of detection and their sensitivity to viral RNA degradation if the cards were to be left in traps for an extended period. Artificially inoculated honey cards were stored at field-like conditions for 1, 3, and 7 days and tested using assays designed to detect WNV (real time RT-PCR, the ProAmpRT WNV LAMP assay, and the RAMP WNV test) and ZIKV (real time RT-PCR and the ProAmpRT ZIKA LAMP assay). The low viral load treatments for the WNV (2.8 log10 PFU) and ZIKV (3.6 log10 PFU) cards are representative of observed expectorate virus in the saliva of Cx. quinquefasciatus and Cx. pipiens infected with WNV (Richards et al. 2012, Fortuna et al. 2015,) and Ae. aegypti infected with ZIKV (Zimler and Alto, unpublished data). The medium and high treatments represent 10-fold and 100-fold greater amounts of virus titer than observed in saliva of these mosquitoes. The real time RT-PCR assay was able to detect virus from cards inoculated with all three virus dilutions held for 1, 3, or 7 days, although the assay exhibited decreased sensitivity with the cards held 7 for days as indicated by an increase in CT values. Similarly, the LAMP assay was successful in detecting virus from cards at all titers and time points, but appeared to be more sensitive to degradation of RNA as fewer positive cards held for 3 and 7 days were detected than when held for 1 day. None of the cards at any time point produced positive results in the RAMP WNV assay. This is not surprising as the RAMP assay has been shown to be less sensitive than real time RT-PCR (Burkhalter et al. 2014).
In the second evaluation, we assessed whether viral RNA may be recovered and detected by real time RT-PCR or the LAMP assay from a honey card fed upon by a single mosquito with a disseminated infection, as previous studies have reported real time RT-PCR results from a single sugar-infused substrate fed upon by several infected mosquitoes (Lothrop et al. 2012). Interestingly, unlike the inoculated cards, there was no significant difference in the real time RT-PCR results of cards held for 1, 3, or 7 days. Very few cards fed upon by WNV-positive mosquitoes (2/25) and none of the cards fed upon by ZIKV-positive mosquitoes produced positive results in the RT-LAMP assay. This is likely due to the low amount of RNA recovered (as indicated by the real time RT-PCR CT values from the same cards) combined with the LAMP assay’s lower sensitivity compared to real time RT-PCR (Burkhalter, unpublished data). According to the real time RT-PCR results, the amount of virus expectorated onto the cards was below the RAMP assay’s previously determined limit of detection (Burkhalter et al. 2014), and thus was excluded from the second evaluation of infected mosquitoes.
Individual infected mosquitoes in this study were allowed ~48 hours to feed on a honey card to mimic most routine vector surveillance protocols in the US that call for traps to be set and collected within 1–2 days. A sugar-feeding rate study performed in Australia found that ~80% of trapped mosquitoes used in that study (Ae. vigilax (Skuse), Cx. annulirostris, and Verrallina funerea (Theobald)) had fed by day 1 (Johnson et al. 2015). Further studies on WNV and ZIKV vectors in the US will be required to determine the optimal trapping length that allows the maximum number mosquitoes to feed on the honey cards, while limiting the degradation of viral RNA.
Taken together these results suggest that to maximize the detection of virus, honey cards should be left in the traps no longer than 1 day if using the ProAmpRT LAMP assays, and no longer than 3 days if using real time RT-PCR. The WNV RAMP assay, which is available to mosquito control districts for pool-based surveillance, is not a likely option for honey card surveillance. Future studies may determine the minimum number of infectious mosquitoes required to feed on a honey card that would be reliably detected by the LAMP or RAMP assays. Real time RT-PCR proved to be the most sensitive of the assays and the most resistant to viral RNA degradation over time, and we expect it will be the optimal assay for detecting arboviruses using this method. Our results also indicate that the honey card surveillance method would be better suited for WNV detection than ZIKV, unless optimization attempts are successful in increasing the sensitivity of ZIKV detection from the cards, regardless of the detection assay used. While a positive honey card result would unequivocally indicate that there are positive mosquitoes in the population, negative results would be harder to interpret. If honey cards are used as an initial screening method, an algorithm may be adopted in which mosquitoes from traps that produce negative cards are pooled and tested by methods optimized for pool-based testing to avoid false-negative results, either due to assay sensitivity limits or if trapped positive mosquitoes did not feed on the substrate.
ACKNOWLEDGEMENTS
We wish to express our gratitude to James Newman at the University of Florida for his assistance in preparing figure 1. We thank Brandy Russell at the Centers for Disease Control and Prevention for the production of Zika virus and Alberto van Olphen for providing the USTOP cards used in this study. This work was supported by the Florida Department of Agriculture and Consumer Services contract number 023592.
REFERENCES CITED
- Alto BW, Wiggins K, Eastmond B, Velez D, Lounibos LP, Lord CC. 2017. Transmission risk of two chikungunya lineages by invasive mosquito vectors from Florida and the Dominican Republic. PLoS Negl Trop Dis 11(17): e0005724 10.1371/journal.pntd.0005724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Connelly RC, O’Meara GF, Hickman D, Karr N. 2014. Reproductive biology and susceptibility of Florida Culex coronator to infection with West Nile virus. Vector-Borne Zoonotic Dis 14:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett-Cadena ND, Gibson J, Lauth M, Stenn T, Acevedo C, Xue RD, McNelly J, Northey E, Hassan HK, Fulcher A, Bingham AM, van Olphen J, van Olphen A, Unnasch TR. 2016. Evaluation of the honey-card technique for detection of transmission of arboviruses in Florida and comparison with sentinel chicken seroconversion. J Med Entomol 53:1449–1457. [DOI] [PubMed] [Google Scholar]
- Burkhalter KL, Horiuchi K, Biggerstaff BJ, Savage HM, Nasci RS. 2014. Evaluation of a rapid analyte measurement platform and real-time reverse-transcriptase polymerase chain reaction assay West Nile virus detection system in mosquito pools. J Am Mosq Contr Assoc 30:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Mendelin S, Ritchie SA, Johansen CA, Zborowski P, Cortis G, Dandridge S, Hall RA, van den Hurk AF. 2010. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc Natl Acad Sci U.S.A 107:11255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. 2013. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 10:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR. 2015. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector Borne Zoonotic Dis 15:397–403. [DOI] [PubMed] [Google Scholar]
- Fortuna C, Remoli ME, Di Luca M, Severini F, Toma L, Benedetti E, Bucci P, Montarsi F, Minelli G, Boccolini D, Romi R, Ciufolini MG. 2015. Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasit Vectors 8:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh ND, Ladner JT, Kraemer MUG, Dudas G, Tan AL, Gangavarapu K, et al. 2017. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 546: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Sánchez S, Cuevas-Romero S, Nemeth NM, Trujillo-Olivera MT, Worwa G, Dupuis A, Brault AC, Kramer LD, Komar N, Estrada-Franco JG. 2011. West Nile virus infection of birds, Mexico. Emerg Infect Dis 17:2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Kerlin T, Hall-Mendelin S, van den Hurk AF, Cortis G, Doggett SL, Toi C, Fali K, McMahon JL, Townsend M, Ritchie SA. 2015. Development and field evaluation of the sentinel mosquito arbovirus capture kit (SMACK). Parasit Vectors 8:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Lee RCH, Chu JJH. 2016. Chikungunya virus: Methods in Biology Molecular. Chu JJH, Ang SK, eds Infectious viral quantification of chikungunya virus – virus plaque assay. New York: Springer Science+Business Media: p. 93–103. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Signor C. 2016. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg Inf Dis 22:933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, and Davis BS. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol 38:4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop HD, Wheeler SS, Fang Y, Reisen WK. 2012. Use of scented sugar bait stations to track mosquito-borne arbovirus transmission in California. J Med Ent 49:1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsky HC, Matranga CB, Wohl S, Schaffner SF, Freije CA, Winnicki SM, et al. 2017. Zika virus evolution and spread in the Americas. Nature 546:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop mediated amplification of DNA. Nucleic Acids Res 28:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Anderson SL, Lord CC, Smartt CT, Tabachnick WJ. 2012. Relationships between infection, dissemination, and transmission of West Nile virus RNA in Culex pipiens quinquefasciatus (Diptera: Culicidae). J Med Ent 49:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, Cortis G, Paton C, Townsend M, Shroyer D, Zborowski P, Hall-Mendelin S, van den Hurk S. 2013. A simple non-powered passive trap for the collection of mosquitoes for arbovirus surveillance. J Med Ent 50:185–194. [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Hall-Mendelin S, Townsend M, Kurucz N, Edwards J, Ehlers G, Rodwell C, Moore FA, McMahon JL, Northill JA, Simmons RJ, Cortis G., Melville L, Whelan PI, Ritchie SA 2014. Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector Borne Zoonotic Dis 14:66–73. [DOI] [PubMed] [Google Scholar]


